Potassium bromate-induced nephrotoxicity and potential curative role of metformin loaded on gold nanoparticles
- PMID: 34293965
- PMCID: PMC10358642
- DOI: 10.1177/00368504211033703
Potassium bromate-induced nephrotoxicity and potential curative role of metformin loaded on gold nanoparticles
Abstract
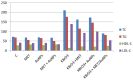
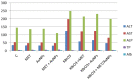
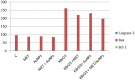
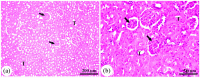
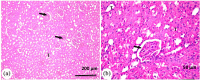
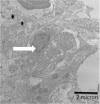
Potassium bromate (KBrO3) is classified by the International Agency for Research on Cancer as a carcinogenic compound, where it causes renal tumors. The present study investigated the potential curative effect of metformin loaded on gold nanoparticles (MET AuNPs) in attenuating KBrO3-induced nephrotoxicity. Rats were divided into eight groups (control, MET, AuNPs, MET AuNPs, KBrO3, KBrO3/MET, KBrO3/AuNPS, and KBrO3/MET AuNPs). KBrO3 administration resulted in a significant elevation in serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total protein (TP), albumin (Alb), total bilirubin (TB), direct bilirubin (DB), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), creatinine, urea, uric acid. Also, KBrO3 significantly increased renal malondialdehyde (MDA), protein carbonyl (PC), and nitric oxide (NO) levels and reduced the activities of antioxidant molecules superoxide dismutase (SOD), catalase (CAT), glutathione-S-transferase (GST), and Reduced glutathione (GSH). It also caused damaged DNA spots in comet assay and increased inflammatory IL-6 and apoptotic markers (caspase 3, Bax) while antiapoptotic Bcl-2 was significantly reduced. MET, AuNPS, MET AuNPS reduced the extent of renal damage induced by KBrO3 as indicated by decreased (AST, ALT, ALP, Alb, TP, TB, DB, creatinine, urea, uric, Lipid profile). MET, AuNPS, MET AuNPS showed a good curative effect against KBrO3-induced nephrotoxicity and MET AuNPS group showed better results compared with monotherapy.
Keywords: Metformin (MET); metformin loaded on gold nanoparticles (MET AuNPs); nephrotoxicity; potassium bromate (KBrO3).
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


















Similar articles
-
Comparative study between effects of ginkgo biloba extract and extract loaded on gold nanoparticles on hepatotoxicity induced by potassium bromate.Environ Sci Pollut Res Int. 2023 Jan;30(2):5027-5036. doi: 10.1007/s11356-022-22324-1. Epub 2022 Aug 17. Environ Sci Pollut Res Int. 2023. PMID: 35978237
-
Effect of Potassium Bromate on the Liver of Adult Male Albino Rat and A Possible Protective Role of Vitamin C: Histological, Immunohistochemical, and Biochemical Study.Anat Rec (Hoboken). 2016 Sep;299(9):1256-69. doi: 10.1002/ar.23386. Epub 2016 Jul 13. Anat Rec (Hoboken). 2016. PMID: 27373450
-
p-Coumaric acid pronounced protective effect against potassium bromate-induced hepatic damage in Swiss albino mice.Cell Biochem Funct. 2024 Jun;42(4):e4076. doi: 10.1002/cbf.4076. Cell Biochem Funct. 2024. PMID: 38895919
-
Toxicity and carcinogenicity of potassium bromate--a new renal carcinogen.Environ Health Perspect. 1990 Jul;87:309-35. doi: 10.1289/ehp.9087309. Environ Health Perspect. 1990. PMID: 2269236 Free PMC article. Review.
-
Preclinical Evaluation of Protective Effects of Terpenoids Against Nanomaterial-Induced Toxicity: A Meta-Analysis.J Appl Toxicol. 2024 Oct 30. doi: 10.1002/jat.4716. Online ahead of print. J Appl Toxicol. 2024. PMID: 39477689 Review.
Cited by
-
Advances in nephroprotection: the therapeutic role of selenium, silver, and gold nanoparticles in renal health.Int Urol Nephrol. 2025 Feb;57(2):479-510. doi: 10.1007/s11255-024-04212-4. Epub 2024 Sep 23. Int Urol Nephrol. 2025. PMID: 39312019 Review.
-
Zinc Oxide Nanoparticles Blunt Potassium-Bromate-Induced Renal Toxicity by Reinforcing the Redox System.Molecules. 2023 Jun 29;28(13):5084. doi: 10.3390/molecules28135084. Molecules. 2023. PMID: 37446744 Free PMC article.
References
-
- Abd El-Rahim AH, Abd-El-Moneim OM, Abd El-Kader HA, et al.. Inhibitory effect of bee venom against potassium bromate causing genetic toxicity and biochemical alterations in mice. J Arab Soc Med Res 2018; 13(2): 89.
-
- Ben Saad H, Driss D, Ellouz Chaabouni S, et al.. Vanillin mitigates potassium bromate-induced molecular, biochemical and histopathological changes in the kidney of adult mice. Chem Biol Interact 2016; 252: 102–113. - PubMed
-
- Nathan DM, Buse JB, Davidson MB, et al.. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32(1): 193–203. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

