N-staging in large cell neuroendocrine carcinoma of the lung: diagnostic value of [18F]FDG PET/CT compared to the histopathology reference standard
- PMID: 34292419
- PMCID: PMC8298649
- DOI: 10.1186/s13550-021-00811-9
N-staging in large cell neuroendocrine carcinoma of the lung: diagnostic value of [18F]FDG PET/CT compared to the histopathology reference standard
Abstract
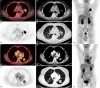
Background: Large cell neuroendocrine carcinoma of the lung (LCNEC) is a rare entity occurring in less than 4% of all lung cancers. Due to its low differentiation and high glucose transporter 1 (GLUT1) expression, LCNEC demonstrates an increased glucose turnover. Thus, PET/CT with 2-[18F]-fluoro-deoxyglucose ([18F]FDG) is suitable for LCNEC staging. Surgery with curative intent is the treatment of choice in early stage LCNEC. Prerequisite for this is correct lymph node staging. This study aimed at evaluating the diagnostic performance of [18F]FDG PET/CT validated by histopathology following surgical resection or mediastinoscopy. N-staging interrater-reliability was assessed to test for robustness of the [18F]FDG PET/CT findings.
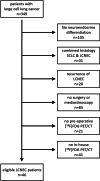
Methods: Between 03/2014 and 12/2020, 46 patients with LCNEC were included in this single center retrospective analysis. All underwent [18F]FDG PET/CT for pre-operative staging and subsequently either surgery (n = 38) or mediastinoscopy (n = 8). Regarding the lymph node involvement, sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV) were calculated for [18F]FDG PET/CT using the final histopathological N-staging (pN0 to pN3) as reference.
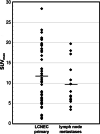
Results: Per patient 14 ± 7 (range 4-32) lymph nodes were resected and histologically processed. 31/46 patients had no LCNEC spread into the lymph nodes. In 8/46 patients, the final stage was pN1, in 5/46 pN2 and in 2/46 pN3. [18F]FDG PET/CT diagnosed lymph node metastasis of LCNEC with a sensitivity of 93%, a specificity of 87%, an accuracy of 89%, a PPV of 78% and a NPV of 96%. In the four false positive cases, the [18F]FDG uptake of the lymph nodes was 33 to 67% less in comparison with that of the respective LCNEC primary. Interrater-reliability was high with a strong level of agreement (κ = 0.82).
Conclusions: In LCNEC N-staging with [18F]FDG PET/CT demonstrates both high sensitivity and specificity, an excellent NPV but a slightly reduced PPV. Accordingly, preoperative invasive mediastinal staging may be omitted in cases with cN0 disease by [18F]FDG PET/CT. In [18F]FDG PET/CT cN1-cN3 stages histological confirmation is warranted, particularly in case of only moderate [18F]FDG uptake as compared to the LCNEC primary.
Keywords: Large cell neuroendocrine carcinoma; Lung; Nodal staging; PET/CT; [18F]FDG.
© 2021. The Author(s).
Conflict of interest statement
Hubertus Hautzel, Yazan Alnajdawi, Christoph Rischpler, Kaid Darwiche, Wilfried E. Eberhardt, Dirk Theegarten, Martin Stuschke, Clemens Aigner and Till Plönes have nothing to disclose. Wolfgang P. Fendler reports personal fees and other from Endocyte, personal fees and other from BTG, personal fees from RadioMedix, personal fees from Bayer Healthcare, personal fees from Parexel, outside the submitted work. Lale Umutlu reports grants, personal fees and other from Siemens Healthineers and personal fees and other from Bayer Healthcare outside the submitted work. Martin Schuler received research funding from AstraZeneca, Boehringer Ingelheim, and Bristol Myers-Squibb and reports personal fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Janssen, MorphoSys, Novartis, Roche, Takeda, Amgen, MSD outside the submitted work. Ken Herrmann reports personal fees from Siemens Healthineers, personal fees from Bayer Healthcare, personal fees and other from Sofie Biosciences, personal fees from SIRTEX, non-financial support from ABX, personal fees from Adacap, personal fees from Curium, personal fees from Endocyte, grants and personal fees from BTG, personal fees from IPSEN, personal fees from GE Healthcare, personal fees from Amgen, personal fees from Novartis, personal fees from ymabs, outside the submitted work.
Figures
Similar articles
-
Diagnostic accuracy of 18F-2-deoxy-fluoro-D-glucose positron emission tomography for pN1 lymph nodes in patients with lung cancer.Acta Radiol. 2009 Jul;50(6):638-44. doi: 10.1080/02841850902971255. Acta Radiol. 2009. PMID: 19492198
-
[18F]FDG PET/CT for assessing inguinal lymph nodes in patients with penile cancer - correlation with histopathology after inguinal lymphadenectomy.Nuklearmedizin. 2018 Feb;57(1):26-30. doi: 10.3412/Nukmed-0932-17-10. Epub 2018 Feb 21. Nuklearmedizin. 2018. PMID: 29536497 English.
-
Mediastinal lymph node staging by FDG-PET in patients with non-small cell lung cancer: analysis of false-positive FDG-PET findings.Respiration. 2003 Sep-Oct;70(5):500-6. doi: 10.1159/000074207. Respiration. 2003. PMID: 14665776
-
Preoperative intrathoracic lymph node staging in patients with non-small-cell lung cancer: accuracy of integrated positron emission tomography and computed tomography.Eur J Cardiothorac Surg. 2009 Sep;36(3):440-5. doi: 10.1016/j.ejcts.2009.04.003. Epub 2009 May 22. Eur J Cardiothorac Surg. 2009. PMID: 19464906 Review.
-
FDG PET-CT for the Detection of Occult Nodal Metastases in Head and Neck Cancer: A Systematic Review and Meta-Analysis.Cancers (Basel). 2024 Aug 24;16(17):2954. doi: 10.3390/cancers16172954. Cancers (Basel). 2024. PMID: 39272812 Free PMC article. Review.
Cited by
-
Neoadjuvant nivolumab with or without relatlimab in resectable non-small-cell lung cancer: a randomized phase 2 trial.Nat Med. 2024 Jun;30(6):1602-1611. doi: 10.1038/s41591-024-02965-0. Epub 2024 Apr 30. Nat Med. 2024. PMID: 38689060 Free PMC article. Clinical Trial.
-
Large Cell Neuroendocrine Carcinoma of the Lung: Current Understanding and Challenges.J Clin Med. 2022 Mar 7;11(5):1461. doi: 10.3390/jcm11051461. J Clin Med. 2022. PMID: 35268551 Free PMC article. Review.
References
-
- Kaira K, Murakami H, Endo M, Ohde Y, Naito T, Kondo H, et al. Biological correlation of 18F-FDG uptake on PET in pulmonary neuroendocrine tumors. Anticancer Res. 2013;33:4219–4228. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

