Membrane association of importin α facilitates viral entry into salivary gland cells of vector insects
- PMID: 34290144
- PMCID: PMC8325321
- DOI: 10.1073/pnas.2103393118
Membrane association of importin α facilitates viral entry into salivary gland cells of vector insects
Abstract
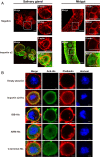
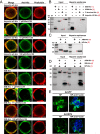
The importin α family belongs to the conserved nuclear transport pathway in eukaryotes. However, the biological functions of importin α in the plasma membrane are still elusive. Here, we report that importin α, as a plasma membrane-associated protein, is exploited by the rice stripe virus (RSV) to enter vector insect cells, especially salivary gland cells. When the expression of three importin α genes was simultaneously knocked down, few virions entered the salivary glands of the small brown planthopper, Laodelphax striatellus Through hemocoel inoculation of virions, only importin α2 was found to efficiently regulate viral entry into insect salivary-gland cells. Importin α2 bound the nucleocapsid protein of RSV with a relatively high affinity through its importin β-binding (IBB) domain, with a dissociation constant KD of 9.1 μM. Furthermore, importin α2 and its IBB domain showed a distinct distribution in the plasma membrane through binding to heparin in heparan sulfate proteoglycan. When the expression of importin α2 was knocked down in viruliferous planthoppers or in nonviruliferous planthoppers before they acquired virions, the viral transmission efficiency of the vector insects in terms of the viral amount and disease incidence in rice was dramatically decreased. These findings not only reveal the specific function of the importin α family in the plasma membrane utilized by viruses, but also provide a promising target gene in vector insects for manipulation to efficiently control outbreaks of rice stripe disease.
Keywords: importin α; plasma membrane; rice stripe virus; salivary gland; small brown planthopper.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
Flotillin 2 Facilitates the Infection of a Plant Virus in the Gut of Insect Vector.J Virol. 2022 Apr 13;96(7):e0214021. doi: 10.1128/jvi.02140-21. Epub 2022 Mar 7. J Virol. 2022. PMID: 35254088 Free PMC article.
-
Different pathogenicities of Rice stripe virus from the insect vector and from viruliferous plants.New Phytol. 2016 Apr;210(1):196-207. doi: 10.1111/nph.13747. Epub 2015 Nov 20. New Phytol. 2016. PMID: 26585422 Free PMC article.
-
The α-tubulin of Laodelphax striatellus mediates the passage of rice stripe virus (RSV) and enhances horizontal transmission.PLoS Pathog. 2020 Aug 20;16(8):e1008710. doi: 10.1371/journal.ppat.1008710. eCollection 2020 Aug. PLoS Pathog. 2020. PMID: 32817722 Free PMC article.
-
The small brown planthopper (Laodelphax striatellus) as a vector of the rice stripe virus.Arch Insect Biochem Physiol. 2023 Feb;112(2):e21992. doi: 10.1002/arch.21992. Epub 2022 Dec 27. Arch Insect Biochem Physiol. 2023. PMID: 36575628 Review.
-
New insights on the transmission mechanism of tenuiviruses by their vector insects.Curr Opin Virol. 2018 Dec;33:13-17. doi: 10.1016/j.coviro.2018.07.004. Epub 2018 Jul 18. Curr Opin Virol. 2018. PMID: 30029017 Review.
Cited by
-
Screening and Characterization of a New Iflavirus Virus in the Fruit Tree Pest Pyrops candelaria.Insects. 2024 Aug 19;15(8):625. doi: 10.3390/insects15080625. Insects. 2024. PMID: 39194829 Free PMC article.
-
Pest status, molecular evolution, and epigenetic factors derived from the genome assembly of Frankliniella fusca, a thysanopteran phytovirus vector.BMC Genomics. 2023 Jun 22;24(1):343. doi: 10.1186/s12864-023-09375-5. BMC Genomics. 2023. PMID: 37344773 Free PMC article.
-
Key role of exportin 6 in exosome-mediated viral transmission from insect vectors to plants.Proc Natl Acad Sci U S A. 2022 Sep 6;119(36):e2207848119. doi: 10.1073/pnas.2207848119. Epub 2022 Aug 29. Proc Natl Acad Sci U S A. 2022. PMID: 36037368 Free PMC article.
-
Nucleo-Cytoplasmic Transport of ZIKV Non-Structural 3 Protein Is Mediated by Importin-α/β and Exportin CRM-1.J Virol. 2023 Jan 31;97(1):e0177322. doi: 10.1128/jvi.01773-22. Epub 2022 Dec 8. J Virol. 2023. PMID: 36475764 Free PMC article.
-
Plasmodesmata-associated Flotillin positively regulates broad-spectrum virus cell-to-cell trafficking.Plant Biotechnol J. 2024 May;22(5):1387-1401. doi: 10.1111/pbi.14274. Epub 2023 Dec 21. Plant Biotechnol J. 2024. PMID: 38130080 Free PMC article.
References
-
- Miyamoto Y., Yamada K., Yoneda Y., Importin α: A key molecule in nuclear transport and non-transport functions. J. Biochem. 160, 69–75 (2016). - PubMed
-
- Miyamoto Y., Boag P. R., Hime G. R., Loveland K. L., Regulated nucleocytoplasmic transport during gametogenesis. Biochim. Biophys. Acta 1819, 616–630 (2012). - PubMed
-
- Goldfarb D. S., Corbett A. H., Mason D. A., Harreman M. T., Adam S. A., Importin α: A multipurpose nuclear-transport receptor. Trends Cell Biol. 14, 505–514 (2004). - PubMed
-
- Chan C. K., Jans D. A., Synergy of importin alpha recognition and DNA binding by the yeast transcriptional activator GAL4. FEBS Lett. 462, 221–224 (1999). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

