Reactive oxygen species in cancer: Current findings and future directions
- PMID: 34286881
- PMCID: PMC8486193
- DOI: 10.1111/cas.15068
Reactive oxygen species in cancer: Current findings and future directions
Abstract
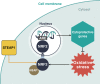
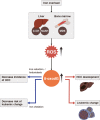
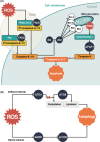
Reactive oxygen species (ROS), a class of highly bioactive molecules, have been widely studied in various types of cancers. ROS are considered to be normal byproducts of numerous cellular processes. Typically, cancer cells exhibit higher basal levels of ROS compared with normal cells as a result of an imbalance between oxidants and antioxidants. ROS have a dual role in cell metabolism: At low to moderate levels, ROS act as signal transducers to activate cell proliferation, migration, invasion, and angiogenesis. In contrast, high levels of ROS cause damage to proteins, nucleic acids, lipids, membranes, and organelles, leading to cell death. Extensive studies have revealed that anticancer therapies that manipulate ROS levels, including immunotherapies, show promising in vitro as well as in vivo results. In this review, we summarize molecular mechanisms and oncogenic functions that modulate ROS levels and are useful for the development of cancer therapeutic strategies. This review also provides insights into the future development of effective agents that regulate the redox system for cancer treatment.
Keywords: cell death; neoplasms; oxidative stress; reactive oxygen species; therapeutics.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors have no conflict of interest.
Figures

Similar articles
-
Recent advances and future directions in etiopathogenesis and mechanisms of reactive oxygen species in cancer treatment.Pathol Oncol Res. 2023 Oct 18;29:1611415. doi: 10.3389/pore.2023.1611415. eCollection 2023. Pathol Oncol Res. 2023. PMID: 37920248 Free PMC article. Review.
-
Activation of apoptosis signalling pathways by reactive oxygen species.Biochim Biophys Acta. 2016 Dec;1863(12):2977-2992. doi: 10.1016/j.bbamcr.2016.09.012. Epub 2016 Sep 17. Biochim Biophys Acta. 2016. PMID: 27646922 Review.
-
Reactive oxygen species in redox cancer therapy.Cancer Lett. 2015 Oct 10;367(1):18-25. doi: 10.1016/j.canlet.2015.07.008. Epub 2015 Jul 14. Cancer Lett. 2015. PMID: 26187782 Review.
-
Reactive Oxygen Species Modulation in the Current Landscape of Anticancer Therapies.Antioxid Redox Signal. 2024 Aug;41(4-6):322-341. doi: 10.1089/ars.2023.0445. Epub 2024 Apr 1. Antioxid Redox Signal. 2024. PMID: 38445392 Review.
-
Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species.Cell Physiol Biochem. 2017;44(2):532-553. doi: 10.1159/000485089. Epub 2017 Nov 17. Cell Physiol Biochem. 2017. PMID: 29145191 Review.
Cited by
-
Kuwanon C Inhibits Tumor Cell Proliferation and Induces Apoptosis by Targeting Mitochondria and Endoplasmic Reticulum.Int J Mol Sci. 2024 Jul 30;25(15):8293. doi: 10.3390/ijms25158293. Int J Mol Sci. 2024. PMID: 39125863 Free PMC article.
-
NoxO1 Determines the Level of ROS Formation by the Nox1-Centered NADPH Oxidase.Antioxidants (Basel). 2024 Sep 14;13(9):1113. doi: 10.3390/antiox13091113. Antioxidants (Basel). 2024. PMID: 39334772 Free PMC article.
-
Innovative Delivery and Release Systems for Antioxidants and Other Active Substances in the Treatment of Cancer.Pharmaceuticals (Basel). 2023 Jul 21;16(7):1038. doi: 10.3390/ph16071038. Pharmaceuticals (Basel). 2023. PMID: 37513948 Free PMC article. Review.
-
Ircinia ramosa Sponge Extract (iSP) Induces Apoptosis in Human Melanoma Cells and Inhibits Melanoma Cell Migration and Invasiveness.Mar Drugs. 2023 Jun 24;21(7):371. doi: 10.3390/md21070371. Mar Drugs. 2023. PMID: 37504902 Free PMC article.
-
Chronic Inflammation, Oxidative Stress and Metabolic Plasticity: Three Players Driving the Pro-Tumorigenic Microenvironment in Malignant Mesothelioma.Cells. 2023 Aug 11;12(16):2048. doi: 10.3390/cells12162048. Cells. 2023. PMID: 37626858 Free PMC article. Review.
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;10:1‐41. - PubMed
-
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85‐95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

