3D Bioprinting Photo-Crosslinkable Hydrogels for Bone and Cartilage Repair
- PMID: 34286152
- PMCID: PMC8287509
- DOI: 10.18063/ijb.v7i3.367
3D Bioprinting Photo-Crosslinkable Hydrogels for Bone and Cartilage Repair
Abstract
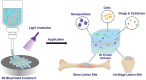
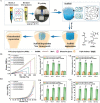
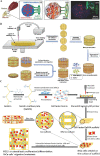
Three-dimensional (3D) bioprinting has become a promising strategy for bone manufacturing, with excellent control over geometry and microarchitectures of the scaffolds. The bioprinting ink for bone and cartilage engineering has thus become the key to developing 3D constructs for bone and cartilage defect repair. Maintaining the balance of cellular viability, drugs or cytokines' function, and mechanical integrity is critical for constructing 3D bone and/or cartilage scaffolds. Photo-crosslinkable hydrogel is one of the most promising materials in tissue engineering; it can respond to light and induce structural or morphological transition. The biocompatibility, easy fabrication, as well as controllable mechanical and degradation properties of photo-crosslinkable hydrogel can meet various requirements of the bone and cartilage scaffolds, which enable it to serve as an effective bio-ink for 3D bioprinting. Here, in this review, we first introduce commonly used photo-crosslinkable hydrogel materials and additives (such as nanomaterials, functional cells, and drugs/cytokine), and then discuss the applications of the 3D bioprinted photo-crosslinkable hydrogel scaffolds for bone and cartilage engineering. Finally, we conclude the review with future perspectives about the development of 3D bioprinting photo-crosslinkable hydrogels in bone and cartilage engineering.
Keywords: Bone and cartilage engineering; Hydrogel; Photo-crosslinking; Three-dimensional printing.
Copyright: © 2021 Mei, et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Photo-crosslinkable methacrylated konjac glucomannan (KGMMA) hydrogels as a promising bioink for 3D bioprinting.Biomater Sci. 2022 Nov 8;10(22):6549-6557. doi: 10.1039/d2bm00832g. Biomater Sci. 2022. PMID: 36205771
-
3D bioprinting of photo-crosslinkable silk methacrylate (SilMA)-polyethylene glycol diacrylate (PEGDA) bioink for cartilage tissue engineering.J Biomed Mater Res A. 2022 Apr;110(4):884-898. doi: 10.1002/jbm.a.37336. Epub 2021 Dec 16. J Biomed Mater Res A. 2022. PMID: 34913587
-
Development of photo-crosslinkable platelet lysate-based hydrogels for 3D printing and tissue engineering.Biofabrication. 2021 Aug 16;13(4). doi: 10.1088/1758-5090/ac1993. Biofabrication. 2021. PMID: 34330124
-
Photo-Crosslinkable Hydrogels for 3D Bioprinting in the Repair of Osteochondral Defects: A Review of Present Applications and Future Perspectives.Micromachines (Basel). 2022 Jun 29;13(7):1038. doi: 10.3390/mi13071038. Micromachines (Basel). 2022. PMID: 35888855 Free PMC article. Review.
-
Shedding light on 3D printing: Printing photo-crosslinkable constructs for tissue engineering.Biomaterials. 2022 Jul;286:121566. doi: 10.1016/j.biomaterials.2022.121566. Epub 2022 May 10. Biomaterials. 2022. PMID: 35633590 Review.
Cited by
-
Smart Bioinks for the Printing of Human Tissue Models.Biomolecules. 2022 Jan 15;12(1):141. doi: 10.3390/biom12010141. Biomolecules. 2022. PMID: 35053289 Free PMC article. Review.
-
Recent Developments and Current Applications of Organic Nanomaterials in Cartilage Repair.Bioengineering (Basel). 2022 Aug 15;9(8):390. doi: 10.3390/bioengineering9080390. Bioengineering (Basel). 2022. PMID: 36004915 Free PMC article. Review.
-
Dynamic Stimulations with Bioengineered Extracellular Matrix-Mimicking Hydrogels for Mechano Cell Reprogramming and Therapy.Adv Sci (Weinh). 2023 Jul;10(21):e2300670. doi: 10.1002/advs.202300670. Epub 2023 Apr 29. Adv Sci (Weinh). 2023. PMID: 37119518 Free PMC article. Review.
-
Properties and Printability of the Synthesized Hydrogel Based on GelMA.Int J Mol Sci. 2023 Jan 20;24(3):2121. doi: 10.3390/ijms24032121. Int J Mol Sci. 2023. PMID: 36768446 Free PMC article.
-
In Situ Photo-crosslinkable Hyaluronic Acid/Gelatin Hydrogel for Local Nitric Oxide Delivery.ACS Appl Mater Interfaces. 2023 Oct 25;15(42):48930-48944. doi: 10.1021/acsami.3c10030. Epub 2023 Oct 12. ACS Appl Mater Interfaces. 2023. PMID: 37827196 Free PMC article.
References
-
- Midha S, Dalela M, Sybil D, et al. Advances in Three-dimensional Bioprinting of Bone:Progress and Challenges. J Tissue Eng Regen Med. 2019;13:925–45. https://doi.org/10.1002/term.2847. - PubMed
-
- Liu Y, Luo D, Wang T. Hierarchical Structures of Bone and Bioinspired Bone Tissue Engineering. Small. 2016;12:4611–32. https://doi.org/10.1002/smll.201600626. - PubMed
-
- Mandrycky C, Wang Z, Kim K, et al. 3D Bioprinting for Engineering Complex Tissues. Biotechnol Adv. 2016;34:422–34. https://doi.org/10.1016/j.biotechadv.2015.12.011. - PMC - PubMed
-
- Allen MR, Burr DB. Basic and Applied Bone Biology. Amsterdam, Netherlands: Elsevier; 2014. Bone Modeling and Remodeling; pp. 75–90. https://doi.org/10.1016/b978-0-12-416015-6.00004-6.
Publication types
LinkOut - more resources
Full Text Sources
