Remdesivir Versus Standard-of-Care for Severe Coronavirus Disease 2019 Infection: An Analysis of 28-Day Mortality
- PMID: 34282406
- PMCID: PMC8244650
- DOI: 10.1093/ofid/ofab278
Remdesivir Versus Standard-of-Care for Severe Coronavirus Disease 2019 Infection: An Analysis of 28-Day Mortality
Abstract
Background: Remdesivir is approved by the US Food and Drug Administration for the treatment of patients hospitalized with coronavirus disease 2019 (COVID-19) and has been shown to shorten time to recovery and improve clinical outcomes in randomized trials.
Methods: This was the final day 28 comparative analysis of data from a phase 3, randomized, open-label study comparing 2 remdesivir regimens (5 vs 10 days, combined for this analysis [remdesivir cohort]) and a real-world retrospective longitudinal cohort study of patients receiving standard-of-care treatment (nonremdesivir cohort). Eligible patients, aged ≥18 years, had confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), oxygen saturation ≤94% on room air or required supplemental oxygen, with pulmonary infiltrates. Propensity score matching (up to 1:10 ratio) was used to ensure comparable populations. We assessed day 14 clinical recovery (determined using a 7-point ordinal scale) and day 28 all-cause mortality (coprimary endpoints).
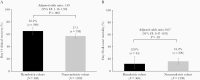
Results: A total of 368 (remdesivir) and 1399 (nonremdesivir) patients were included in the matched analysis. The day 14 clinical recovery rate was significantly higher among the remdesivir versus the nonremdesivir cohort (65.2% vs 57.1%; odds ratio [OR], 1.49; 95% confidence interval [CI], 1.16-1.90; P = 0.002). The day 28 mortality rate was significantly lower in the remdesivir cohort versus the nonremdesivir cohort (12.0% vs 16.2%; OR, 0.67; 95% CI, 0.47-.95; P = .03).
Conclusions: Remdesivir was associated with significantly higher rates of day 14 clinical recovery, and lower day 28 mortality, compared with standard-of-care treatment in hospitalized patients with COVID-19. These data, taken together, support the use of remdesivir to improve clinical recovery and decrease mortality from SARS-CoV-2 infection.
Keywords: COVID-19; SARS-CoV-2; mortality; remdesivir.
© The Author(s) 2021. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures


Similar articles
-
Remdesivir for Severe Coronavirus Disease 2019 (COVID-19) Versus a Cohort Receiving Standard of Care.Clin Infect Dis. 2021 Dec 6;73(11):e4166-e4174. doi: 10.1093/cid/ciaa1041. Clin Infect Dis. 2021. PMID: 32706859 Free PMC article. Clinical Trial.
-
Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial.JAMA. 2020 Sep 15;324(11):1048-1057. doi: 10.1001/jama.2020.16349. JAMA. 2020. PMID: 32821939 Free PMC article. Clinical Trial.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Remdesivir for the Treatment of COVID-19: A Narrative Review.Infect Dis Ther. 2024 Jan;13(1):1-19. doi: 10.1007/s40121-023-00900-3. Epub 2024 Jan 9. Infect Dis Ther. 2024. PMID: 38193988 Free PMC article. Review.
-
Efficacy and safety of remdesivir in hospitalized Covid-19 patients: Systematic review and meta-analysis including network meta-analysis.Rev Med Virol. 2021 Jul;31(4):e2187. doi: 10.1002/rmv.2187. Epub 2020 Oct 31. Rev Med Virol. 2021. PMID: 33128490 Review.
Cited by
-
The efficacy and safety of remdesivir alone and in combination with other drugs for the treatment of COVID-19: a systematic review and meta-analysis.BMC Infect Dis. 2023 Oct 9;23(1):672. doi: 10.1186/s12879-023-08525-0. BMC Infect Dis. 2023. PMID: 37814214 Free PMC article.
-
Effectiveness and Safety of Remdesivir in Treating Hospitalised Patients with COVID-19: A Propensity Score Analysis of Real-Life Data from a Monocentric Observational Study in Times of Health Emergency.Clin Drug Investig. 2023 Oct;43(10):763-771. doi: 10.1007/s40261-023-01304-4. Epub 2023 Sep 22. Clin Drug Investig. 2023. PMID: 37740148
-
From Archipelago to Pandemic Battleground: Unveiling Indonesia's COVID-19 Crisis.J Epidemiol Glob Health. 2023 Dec;13(4):591-603. doi: 10.1007/s44197-023-00148-7. Epub 2023 Sep 14. J Epidemiol Glob Health. 2023. PMID: 37707715 Free PMC article. Review.
-
Remdesivir: A Review in COVID-19.Drugs. 2023 Sep;83(13):1215-1237. doi: 10.1007/s40265-023-01926-0. Epub 2023 Aug 17. Drugs. 2023. PMID: 37589788 Free PMC article. Review.
-
Remdesivir Treatment Lacks the Effect on Mortality Reduction in Hospitalized Adult COVID-19 Patients Who Required High-Flow Supplemental Oxygen or Invasive Mechanical Ventilation.Medicina (Kaunas). 2023 May 26;59(6):1027. doi: 10.3390/medicina59061027. Medicina (Kaunas). 2023. PMID: 37374231 Free PMC article. Review.
References
-
- Johns Hopkins University and Medicine. Coronavirus resource center. Available at: https://coronavirus.jhu.edu/. Accessed 17 February 2021.
-
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020; 324:782–93. - PubMed

