ARVib suppresses growth of advanced prostate cancer via inhibition of androgen receptor signaling
- PMID: 34272475
- PMCID: PMC8413131
- DOI: 10.1038/s41388-021-01914-2
ARVib suppresses growth of advanced prostate cancer via inhibition of androgen receptor signaling
Abstract
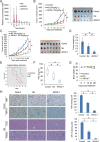
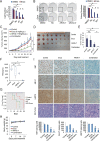
Targeting androgen signaling with the second-generation anti-androgen drugs, such as enzalutamide (Enza), abiraterone (Abi), apalutamide (Apal), and darolutamide (Daro), is the mainstay for the treatment of castration-resistant prostate cancer (CRPC). While these treatments are effective initially, resistance occurs frequently. Continued expression of androgen receptor (AR) and its variants such as AR-V7 despite AR-targeted therapy contributes to treatment resistance and cancer progression in advanced CRPC patients. This highlights the need for new strategies blocking continued AR signaling. Here, we identify a novel AR/AR-V7 degrader (ARVib) and found that ARVib effectively degrades AR/AR-V7 protein and attenuates AR/AR-V7 downstream target gene expression in prostate cancer cells. Mechanistically, ARVib degrades AR/AR-V7 protein through the ubiquitin-proteasome pathway mediated by HSP70/STUB1 machinery modulation. ARVib suppresses HSP70 expression and promotes STUB1 nuclear translocation, where STUB1 binds to AR/AR-V7 and promotes its ubiquitination and degradation. ARVib significantly inhibits resistant prostate tumor growth and improves enzalutamide treatment in vitro and in vivo. These data suggest that ARVib has potential for development as an AR/AR-V7 degrader to treat resistant CRPC.
© 2021. The Author(s).
Conflict of interest statement
PKL and ACG are co-inventors of a patent application of the small molecule inhibitors of androgen receptor variants (ARVib). All other authors declare no competing interests.
Figures
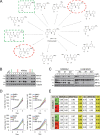
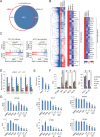
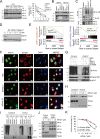
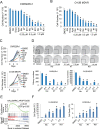
Comment in
-
ARVib inhibits signalling to suppress growth.Nat Rev Urol. 2021 Sep;18(9):510. doi: 10.1038/s41585-021-00509-6. Nat Rev Urol. 2021. PMID: 34312529 No abstract available.
Similar articles
-
TAS3681, an androgen receptor antagonist, prevents drug resistance driven by aberrant androgen receptor signaling in prostate cancer.Mol Oncol. 2024 Aug;18(8):1980-2000. doi: 10.1002/1878-0261.13641. Epub 2024 Apr 10. Mol Oncol. 2024. PMID: 38600681 Free PMC article.
-
Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer.Clin Cancer Res. 2014 Jun 15;20(12):3198-3210. doi: 10.1158/1078-0432.CCR-13-3296. Epub 2014 Apr 16. Clin Cancer Res. 2014. PMID: 24740322 Free PMC article.
-
Targeted Delivery of AR-V7 siRNA with Bivalent PSMA Aptamers Effectively Suppresses the Growth of Enzalutamide-Resistant Prostate Cancer.Mol Pharm. 2024 Nov 4;21(11):5749-5760. doi: 10.1021/acs.molpharmaceut.4c00743. Epub 2024 Oct 10. Mol Pharm. 2024. PMID: 39388218
-
Second-Generation Androgen Receptor Antagonists as Hormonal Therapeutics for Three Forms of Prostate Cancer.Molecules. 2020 May 24;25(10):2448. doi: 10.3390/molecules25102448. Molecules. 2020. PMID: 32456317 Free PMC article. Review.
-
Androgen receptor splice variants in the era of enzalutamide and abiraterone.Horm Cancer. 2014 Oct;5(5):265-73. doi: 10.1007/s12672-014-0190-1. Epub 2014 Jul 22. Horm Cancer. 2014. PMID: 25048254 Free PMC article. Review.
Cited by
-
A compendium of Androgen Receptor Variant 7 target genes and their role in Castration Resistant Prostate Cancer.Front Oncol. 2023 Mar 1;13:1129140. doi: 10.3389/fonc.2023.1129140. eCollection 2023. Front Oncol. 2023. PMID: 36937454 Free PMC article. Review.
-
Targeting Inflammatory Signaling in Prostate Cancer Castration Resistance.J Clin Med. 2021 Oct 27;10(21):5000. doi: 10.3390/jcm10215000. J Clin Med. 2021. PMID: 34768524 Free PMC article. Review.
-
Extracellular Vesicles in Advanced Prostate Cancer: Tools to Predict and Thwart Therapeutic Resistance.Cancers (Basel). 2021 Jul 28;13(15):3791. doi: 10.3390/cancers13153791. Cancers (Basel). 2021. PMID: 34359692 Free PMC article. Review.
-
Constitutively Active Androgen Receptor in Hepatocellular Carcinoma.Int J Mol Sci. 2022 Nov 9;23(22):13768. doi: 10.3390/ijms232213768. Int J Mol Sci. 2022. PMID: 36430245 Free PMC article. Review.
-
The crosstalk between ubiquitination and endocrine therapy.J Mol Med (Berl). 2023 May;101(5):461-486. doi: 10.1007/s00109-023-02300-z. Epub 2023 Mar 24. J Mol Med (Berl). 2023. PMID: 36961537 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

