Interactions between semaphorins and plexin-neuropilin receptor complexes in the membranes of live cells
- PMID: 34270956
- PMCID: PMC8350011
- DOI: 10.1016/j.jbc.2021.100965
Interactions between semaphorins and plexin-neuropilin receptor complexes in the membranes of live cells
Abstract
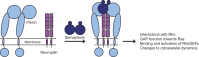
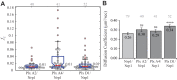
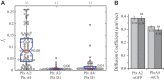
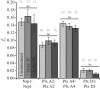
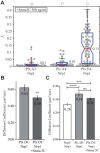
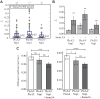
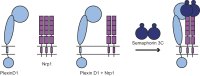
Signaling of semaphorin ligands via their plexin-neuropilin receptors is involved in tissue patterning in the developing embryo. These proteins play roles in cell migration and adhesion but are also important in disease etiology, including in cancer angiogenesis and metastasis. While some structures of the soluble domains of these receptors have been determined, the conformations of the full-length receptor complexes are just beginning to be elucidated, especially within the context of the plasma membrane. Pulsed-interleaved excitation fluorescence cross-correlation spectroscopy allows direct insight into the formation of protein-protein interactions in the membranes of live cells. Here, we investigated the homodimerization of neuropilin-1 (Nrp1), plexin A2, plexin A4, and plexin D1 using pulsed-interleaved excitation fluorescence cross-correlation spectroscopy. Consistent with previous studies, we found that Nrp1, plexin A2, and plexin A4 are present as dimers in the absence of exogenous ligand. Plexin D1, on the other hand, was monomeric under similar conditions, which had not been previously reported. We also found that plexin A2 and A4 assemble into a heteromeric complex. Stimulation with semaphorin 3A or semaphorin 3C neither disrupts nor enhances the dimerization of the receptors when expressed alone, suggesting that activation involves a conformational change rather than a shift in the monomer-dimer equilibrium. However, upon stimulation with semaphorin 3C, plexin D1 and Nrp1 form a heteromeric complex. This analysis of interactions provides a complementary approach to the existing structural and biochemical data that will aid in the development of new therapeutic strategies to target these receptors in cancer.
Keywords: cancer; cell signaling; fluorescence correlation spectroscopy; membrane biophysics; membrane protein; protein–protein interaction.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

Similar articles
-
Class A Plexins Are Organized as Preformed Inactive Dimers on the Cell Surface.Biophys J. 2015 Nov 3;109(9):1937-45. doi: 10.1016/j.bpj.2015.04.043. Biophys J. 2015. PMID: 26536270 Free PMC article.
-
Transmembrane recognition of the semaphorin co-receptors neuropilin 1 and plexin A1: coarse-grained simulations.PLoS One. 2014 May 23;9(5):e97779. doi: 10.1371/journal.pone.0097779. eCollection 2014. PLoS One. 2014. PMID: 24858828 Free PMC article.
-
The role of the plexin-A2 receptor in Sema3A and Sema3B signal transduction.J Cell Sci. 2014 Dec 15;127(Pt 24):5240-52. doi: 10.1242/jcs.155960. Epub 2014 Oct 21. J Cell Sci. 2014. PMID: 25335892
-
Plexin structures are coming: opportunities for multilevel investigations of semaphorin guidance receptors, their cell signaling mechanisms, and functions.Cell Mol Life Sci. 2012 Nov;69(22):3765-805. doi: 10.1007/s00018-012-1019-0. Epub 2012 Jun 29. Cell Mol Life Sci. 2012. PMID: 22744749 Free PMC article. Review.
-
Current drug design to target the Semaphorin/Neuropilin/Plexin complexes.Cell Adh Migr. 2016 Nov;10(6):700-708. doi: 10.1080/19336918.2016.1261785. Cell Adh Migr. 2016. PMID: 27906605 Free PMC article. Review.
Cited by
-
Single-virus tracking reveals variant SARS-CoV-2 spike proteins induce ACE2-independent membrane interactions.Sci Adv. 2022 Dec 9;8(49):eabo3977. doi: 10.1126/sciadv.abo3977. Epub 2022 Dec 9. Sci Adv. 2022. PMID: 36490345 Free PMC article.
-
Regulation of Semaphorin3A in the process of cutaneous wound healing.Cell Death Differ. 2022 Oct;29(10):1941-1954. doi: 10.1038/s41418-022-00981-6. Epub 2022 Mar 26. Cell Death Differ. 2022. PMID: 35347234 Free PMC article. Review.
-
SEMA7A-mediated juxtacrine stimulation of IGFBP-3 upregulates IL-17RB at pancreatic cancer invasive front.Cancer Gene Ther. 2024 Dec;31(12):1840-1855. doi: 10.1038/s41417-024-00849-6. Epub 2024 Oct 24. Cancer Gene Ther. 2024. PMID: 39448803 Free PMC article.
-
SEMA3B inhibits TGFβ-induced extracellular matrix protein production and its reduced levels are associated with a decline in lung function in IPF.Am J Physiol Cell Physiol. 2024 Jun 1;326(6):C1659-C1668. doi: 10.1152/ajpcell.00681.2023. Epub 2024 Apr 22. Am J Physiol Cell Physiol. 2024. PMID: 38646784 Free PMC article.
-
Motor neurons use push-pull signals to direct vascular remodeling critical for their connectivity.Neuron. 2022 Dec 21;110(24):4090-4107.e11. doi: 10.1016/j.neuron.2022.09.021. Epub 2022 Oct 13. Neuron. 2022. PMID: 36240771 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

