Homo-oligomerization of the human adenosine A2A receptor is driven by the intrinsically disordered C-terminus
- PMID: 34269678
- PMCID: PMC8328514
- DOI: 10.7554/eLife.66662
Homo-oligomerization of the human adenosine A2A receptor is driven by the intrinsically disordered C-terminus
Abstract
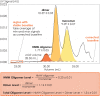
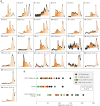
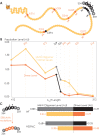
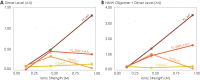
G protein-coupled receptors (GPCRs) have long been shown to exist as oligomers with functional properties distinct from those of the monomeric counterparts, but the driving factors of oligomerization remain relatively unexplored. Herein, we focus on the human adenosine A2A receptor (A2AR), a model GPCR that forms oligomers both in vitro and in vivo. Combining experimental and computational approaches, we discover that the intrinsically disordered C-terminus of A2AR drives receptor homo-oligomerization. The formation of A2AR oligomers declines progressively with the shortening of the C-terminus. Multiple interaction types are responsible for A2AR oligomerization, including disulfide linkages, hydrogen bonds, electrostatic interactions, and hydrophobic interactions. These interactions are enhanced by depletion interactions, giving rise to a tunable network of bonds that allow A2AR oligomers to adopt multiple interfaces. This study uncovers the disordered C-terminus as a prominent driving factor for the oligomerization of a GPCR, offering important insight into the effect of C-terminus modification on receptor oligomerization of A2AR and other GPCRs reconstituted in vitro for biophysical studies.
Keywords: E. coli; S. cerevisiae; adenosine a2a receptor; biochemistry; c-terminus; chemical biology; depletion interactions; gpcrs; intrinsically disordered regions; molecular biophysics; oligomerization; structural biology.
© 2021, Nguyen et al.
Conflict of interest statement
KN, MV, ES, SS, JH, NS, BM, MO, SH No competing interests declared
Figures





Similar articles
-
Modulation of adenosine A2a receptor oligomerization by receptor activation and PIP2 interactions.Structure. 2021 Nov 4;29(11):1312-1325.e3. doi: 10.1016/j.str.2021.06.015. Epub 2021 Jul 15. Structure. 2021. PMID: 34270937 Free PMC article.
-
Calcium modulates calmodulin/α-actinin 1 interaction with and agonist-dependent internalization of the adenosine A2A receptor.Biochim Biophys Acta Mol Cell Res. 2017 Apr;1864(4):674-686. doi: 10.1016/j.bbamcr.2017.01.013. Epub 2017 Jan 24. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 28130124
-
Revealing Adenosine A2A-Dopamine D2 Receptor Heteromers in Parkinson's Disease Post-Mortem Brain through a New AlphaScreen-Based Assay.Int J Mol Sci. 2019 Jul 23;20(14):3600. doi: 10.3390/ijms20143600. Int J Mol Sci. 2019. PMID: 31340557 Free PMC article.
-
Novel approaches for targeting the adenosine A2A receptor.Expert Opin Drug Discov. 2015 Jan;10(1):63-80. doi: 10.1517/17460441.2015.971006. Epub 2014 Oct 14. Expert Opin Drug Discov. 2015. PMID: 25311639 Review.
-
Evidence for the heterotetrameric structure of the adenosine A2A-dopamine D2 receptor complex.Biochem Soc Trans. 2016 Apr 15;44(2):595-600. doi: 10.1042/BST20150276. Biochem Soc Trans. 2016. PMID: 27068975 Review.
Cited by
-
Membrane Heteroreceptor Complexes as Second-Order Protein Modulators: A Novel Integrative Mechanism through Allosteric Receptor-Receptor Interactions.Membranes (Basel). 2024 Apr 25;14(5):96. doi: 10.3390/membranes14050096. Membranes (Basel). 2024. PMID: 38786931 Free PMC article.
-
Modulation of adenosine A2a receptor oligomerization by receptor activation and PIP2 interactions.Structure. 2021 Nov 4;29(11):1312-1325.e3. doi: 10.1016/j.str.2021.06.015. Epub 2021 Jul 15. Structure. 2021. PMID: 34270937 Free PMC article.
-
Combined treatment with Sigma1R and A2AR agonists fails to inhibit cocaine self-administration despite causing strong antagonistic accumbal A2AR-D2R complex interactions: the potential role of astrocytes.Front Mol Neurosci. 2023 May 24;16:1106765. doi: 10.3389/fnmol.2023.1106765. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37293542 Free PMC article.
-
Genetic tagging of the adenosine A2A receptor reveals its heterogeneous expression in brain regions.Front Neuroanat. 2022 Aug 18;16:978641. doi: 10.3389/fnana.2022.978641. eCollection 2022. Front Neuroanat. 2022. PMID: 36059431 Free PMC article.
-
Yeast-based directed-evolution for high-throughput structural stabilization of G protein-coupled receptors (GPCRs).Sci Rep. 2022 May 23;12(1):8657. doi: 10.1038/s41598-022-12731-2. Sci Rep. 2022. PMID: 35606532 Free PMC article.
References
-
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1-2:19–25. doi: 10.1016/j.softx.2015.06.001. - DOI
-
- Armando S, Quoyer J, Lukashova V, Maiga A, Percherancier Y, Heveker N, Pin JP, Prézeau L, Bouvier M. The chemokine CXC4 and CC2 receptors form Homo- and heterooligomers that can engage their signaling G-protein effectors and βarrestin. The FASEB Journal. 2014;28:4509–4523. doi: 10.1096/fj.13-242446. - DOI - PubMed
-
- Asakura S, Oosawa F. Interaction between particles suspended in solutions of macromolecules. Journal of Polymer Science. 1958;33:183–192. doi: 10.1002/pol.1958.1203312618. - DOI

