Real-World Effectiveness of Adjuvant Oxaliplatin Chemotherapy in Stage III Colon Cancer: A Controlled Interrupted Time Series Analysis
- PMID: 34267662
- PMCID: PMC8276019
- DOI: 10.3389/fphar.2021.693009
Real-World Effectiveness of Adjuvant Oxaliplatin Chemotherapy in Stage III Colon Cancer: A Controlled Interrupted Time Series Analysis
Abstract
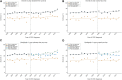
Background: The real-world effectiveness of oxaliplatin in stage III colon cancer has not been determined in a large-scale population. We aimed to assess the real-world impact of adjuvant oxaliplatin treatment on the survival of these patients. Methods: Based on Taiwan cancer registry, we evaluated 17,801 patients with resected stage III colon cancer, including 14,168 patients receiving adjuvant chemotherapy and 3,633 not receiving adjuvant chemotherapy as the control group between 2004 and 2014. We used the controlled interrupted time-series analysis to assess the three-year disease-free survival and five-year overall survival rates before (2004-2008) and after (2009-2014) the addition of oxaliplatin. Results: The introduction of oxaliplatin was associated with no significant improvement in the slopes (per half-year) of the three-year disease-free survival rate (0.2%, 95% CI: -1.7∼2.2%) and five-year overall survival rate (0.6%, 95% CI: -1.8∼3%). The patients receiving oxaliplatin-based chemotherapy also showed no significant increase in the slopes (per half-year) of the three-year disease-free survival rate (0.6%, 95% CI: -1.4∼2.6%) and five-year overall survival rate (1%, 95% CI: -1.5∼3.5%). The nonsignificant results were consistent across subgroup analyses of age (<70 vs. ≥70 years), recurrence risk (T1-3 or N1 vs. T4 or N2), and cycle of oxaliplatin use (≤6 vs. >6). However, oxaliplatin-based chemotherapy significantly increased the slope (per half-year) of the five-year OS (2%, 95% CI: 0.2∼3.8%) for patients in the high-risk group (T4 or N2). The present results were robust in several sensitivity analyses. Conclusion: Among real-world patients with stage III colon cancer, the introduction of oxaliplatin does not yield a significant improvement in survival. Future work should identify the subpopulation(s) of patients who benefit significantly from the addition of oxaliplatin.
Keywords: adjuvant chemotherapy; interrupted time series study; mortality; oxaliplatin; stage III colorectal cancer.
Copyright © 2021 Huang, Hsu, Chang, Chou, Chang, Chiang, Chang, Chen, Yang and See.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Assessment of Duration and Effects of 3 vs 6 Months of Adjuvant Chemotherapy in High-Risk Stage II Colorectal Cancer: A Subgroup Analysis of the TOSCA Randomized Clinical Trial.JAMA Oncol. 2020 Apr 1;6(4):547-551. doi: 10.1001/jamaoncol.2019.6486. JAMA Oncol. 2020. PMID: 32053133 Free PMC article. Clinical Trial.
-
Three Versus 6 Months of Oxaliplatin-Based Adjuvant Chemotherapy for Patients With Stage III Colon Cancer: Disease-Free Survival Results From a Randomized, Open-Label, International Duration Evaluation of Adjuvant (IDEA) France, Phase III Trial.J Clin Oncol. 2018 May 20;36(15):1469-1477. doi: 10.1200/JCO.2017.76.0355. Epub 2018 Apr 5. J Clin Oncol. 2018. PMID: 29620995 Clinical Trial.
-
Association of Bevacizumab Plus Oxaliplatin-Based Chemotherapy With Disease-Free Survival and Overall Survival in Patients With Stage II Colon Cancer: A Secondary Analysis of the AVANT Trial.JAMA Netw Open. 2020 Oct 1;3(10):e2020425. doi: 10.1001/jamanetworkopen.2020.20425. JAMA Netw Open. 2020. PMID: 33074326 Free PMC article. Clinical Trial.
-
The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation.Health Technol Assess. 2006 Nov;10(41):iii-iv, xi-xiv, 1-185. doi: 10.3310/hta10410. Health Technol Assess. 2006. PMID: 17049138 Review.
-
A systematic overview of chemotherapy effects in colorectal cancer.Acta Oncol. 2001;40(2-3):282-308. doi: 10.1080/02841860151116367. Acta Oncol. 2001. PMID: 11441937 Review.
Cited by
-
Incidence of oxaliplatin hypersensitivity reaction among colorectal cancer patients: A 5-year retrospective study.Pharm Pract (Granada). 2022 Apr-Jun;20(2):2635. doi: 10.18549/PharmPract.2022.2.2635. Epub 2022 Mar 31. Pharm Pract (Granada). 2022. PMID: 35919789 Free PMC article.
-
Can immunotherapy reinforce chemotherapy efficacy? a new perspective on colorectal cancer treatment.Front Immunol. 2023 Sep 18;14:1237764. doi: 10.3389/fimmu.2023.1237764. eCollection 2023. Front Immunol. 2023. PMID: 37790928 Free PMC article. Review.
-
Dual-targeting peptides@PMO, a mimetic to the pro-apoptotic protein Smac/DIABLO for selective activation of apoptosis in cancer cells.Front Pharmacol. 2023 Aug 29;14:1237478. doi: 10.3389/fphar.2023.1237478. eCollection 2023. Front Pharmacol. 2023. PMID: 37711175 Free PMC article.
References
-
- André T., De Gramont A., Vernerey D., Chibaudel B., Bonnetain F., Tijeras-Raballand A., et al. (2015). Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. Jco 33, 4176–4187. 10.1200/JCO.2015.63.4238 - DOI - PubMed
LinkOut - more resources
Full Text Sources

