Fixed-target serial femtosecond crystallography using in cellulo grown microcrystals
- PMID: 34258014
- PMCID: PMC8256716
- DOI: 10.1107/S2052252521005297
Fixed-target serial femtosecond crystallography using in cellulo grown microcrystals
Abstract
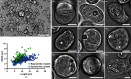
The crystallization of recombinant proteins in living cells is an exciting new approach in structural biology. Recent success has highlighted the need for fast and efficient diffraction data collection, optimally directly exposing intact crystal-containing cells to the X-ray beam, thus protecting the in cellulo crystals from environmental challenges. Serial femtosecond crystallography (SFX) at free-electron lasers (XFELs) allows the collection of detectable diffraction even from tiny protein crystals, but requires very fast sample exchange to utilize each XFEL pulse. Here, an efficient approach is presented for high-resolution structure elucidation using serial femtosecond in cellulo diffraction of micometre-sized crystals of the protein HEX-1 from the fungus Neurospora crassa on a fixed target. Employing the fast and highly accurate Roadrunner II translation-stage system allowed efficient raster scanning of the pores of micro-patterned, single-crystalline silicon chips loaded with living, crystal-containing insect cells. Compared with liquid-jet and LCP injection systems, the increased hit rates of up to 30% and reduced background scattering enabled elucidation of the HEX-1 structure. Using diffraction data from only a single chip collected within 12 min at the Linac Coherent Light Source, a 1.8 Å resolution structure was obtained with significantly reduced sample consumption compared with previous SFX experiments using liquid-jet injection. This HEX-1 structure is almost superimposable with that previously determined using synchrotron radiation from single HEX-1 crystals grown by sitting-drop vapour diffusion, validating the approach. This study demonstrates that fixed-target SFX using micro-patterned silicon chips is ideally suited for efficient in cellulo diffraction data collection using living, crystal-containing cells, and offers huge potential for the straightforward structure elucidation of proteins that form intracellular crystals at both XFELs and synchrotron sources.
Keywords: Roadrunner; fixed-target SFX; in cellulo crystallography; intracellular protein crystals; serial femtosecond crystallography; silicon chip.
© J. Mia Lahey-Rudolph et al. 2021.
Figures


Similar articles
-
Preparation and Delivery of Protein Microcrystals in Lipidic Cubic Phase for Serial Femtosecond Crystallography.J Vis Exp. 2016 Sep 20;(115):54463. doi: 10.3791/54463. J Vis Exp. 2016. PMID: 27683972 Free PMC article.
-
Serial femtosecond crystallography of soluble proteins in lipidic cubic phase.IUCrJ. 2015 Aug 4;2(Pt 5):545-51. doi: 10.1107/S2052252515013160. eCollection 2015 Sep 1. IUCrJ. 2015. PMID: 26306196 Free PMC article.
-
On-chip crystallization for serial crystallography experiments and on-chip ligand-binding studies.IUCrJ. 2019 Jun 19;6(Pt 4):714-728. doi: 10.1107/S2052252519007395. eCollection 2019 Jul 1. IUCrJ. 2019. PMID: 31316815 Free PMC article.
-
Femtosecond crystallography of membrane proteins in the lipidic cubic phase.Philos Trans R Soc Lond B Biol Sci. 2014 Jul 17;369(1647):20130314. doi: 10.1098/rstb.2013.0314. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 24914147 Free PMC article. Review.
-
A guide to sample delivery systems for serial crystallography.FEBS J. 2019 Nov;286(22):4402-4417. doi: 10.1111/febs.15099. Epub 2019 Nov 6. FEBS J. 2019. PMID: 31618529 Review.
Cited by
-
Advanced manufacturing provides tailor-made solutions for crystallography with x-ray free-electron lasers.Struct Dyn. 2024 Feb 21;11(1):011101. doi: 10.1063/4.0000229. eCollection 2024 Jan. Struct Dyn. 2024. PMID: 38389979 Free PMC article.
-
Bridging the microscopic divide: a comprehensive overview of micro-crystallization and in vivo crystallography.IUCrJ. 2024 Jul 1;11(Pt 4):476-485. doi: 10.1107/S205225252400513X. IUCrJ. 2024. PMID: 38958014 Free PMC article. Review.
-
Convolutional neural network approach for the automated identification of in cellulo crystals.J Appl Crystallogr. 2024 Feb 23;57(Pt 2):266-275. doi: 10.1107/S1600576724000682. eCollection 2024 Apr 1. J Appl Crystallogr. 2024. PMID: 38596734 Free PMC article.
-
A simple vapor-diffusion method enables protein crystallization inside the HARE serial crystallography chip.Acta Crystallogr D Struct Biol. 2021 Jun 1;77(Pt 6):820-834. doi: 10.1107/S2059798321003855. Epub 2021 May 19. Acta Crystallogr D Struct Biol. 2021. PMID: 34076595 Free PMC article.
-
Review of serial femtosecond crystallography including the COVID-19 pandemic impact and future outlook.Structure. 2023 Nov 2;31(11):1306-1319. doi: 10.1016/j.str.2023.10.005. Epub 2023 Oct 27. Structure. 2023. PMID: 37898125 Free PMC article. Review.
References
-
- Ayyer, K., Yefanov, O. M., Oberthür, D., Roy-Chowdhury, S., Galli, L., Mariani, V., Basu, S., Coe, J., Conrad, C. E., Fromme, R., Schaffer, A., Dörner, K., James, D., Kupitz, C., Metz, M., Nelson, G., Xavier, P. L., Beyerlein, K. R., Schmidt, M., Sarrou, I., Spence, J. C. H., Weierstall, U., White, T. A., Yang, J.-H., Zhao, Y., Liang, M., Aquila, A., Hunter, M. S., Robinson, J. S., Koglin, J. E., Boutet, S., Fromme, P., Barty, A. & Chapman, H. N. (2016). Nature, 530, 202–206. - PMC - PubMed
-
- Baxter, E. L., Aguila, L., Alonso-Mori, R., Barnes, C. O., Bonagura, C. A., Brehmer, W., Brunger, A. T., Calero, G., Caradoc-Davies, T. T., Chatterjee, R., Degrado, W. F., Fraser, J. M., Ibrahim, M., Kern, J., Kobilka, B. K., Kruse, A. C., Larsson, K. M., Lemke, H. T., Lyubimov, A. Y., Manglik, A., McPhillips, S. E., Norgren, E., Pang, S. S., Soltis, S. M., Song, J., Thomaston, J., Tsai, Y., Weis, W. I., Woldeyes, R. A., Yachandra, V., Yano, J., Zouni, A. & Cohen, A. E. (2016). Acta Cryst. D72, 2–11. - PubMed
-
- Beyerlein, K. R., Dierksmeyer, D., Mariani, V., Kuhn, M., Sarrou, I., Ottaviano, A., Awel, S., Knoska, J., Fuglerud, S., Jönsson, O., Stern, S., Wiedorn, M. O., Yefanov, O., Adriano, L., Bean, R., Burkhardt, A., Fischer, P., Heymann, M., Horke, D. A., Jungnickel, K. E. J., Kovaleva, E., Lorbeer, O., Metz, M., Meyer, J., Morgan, A., Pande, K., Panneerselvam, S., Seuring, C., Tolstikova, A., Lieske, J., Aplin, S., Roessle, M., White, T. A., Chapman, H. N., Meents, A. & Oberthuer, D. (2017). IUCrJ, 4, 769–777. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

