Axillary dissection versus axillary observation for low risk, clinically node-negative invasive breast cancer: a systematic review and meta-analysis
- PMID: 34241800
- PMCID: PMC8514376
- DOI: 10.1007/s12282-021-01273-6
Axillary dissection versus axillary observation for low risk, clinically node-negative invasive breast cancer: a systematic review and meta-analysis
Abstract
Purpose: 1. To systematically analyse studies comparing survival outcomes between axillary lymph-node dissection (ALND) and axilla observation (Obs), in women with low-risk, clinically node-negative breast cancer. 2. To consider results in the context of current axillary surgery de-escalation trials and studies.
Methods: 9 eligible studies were identified, 6 RCTs and 3 non-randomized studies (4236 women in total). Outcomes assessed: overall survival (OS) and disease-free survival (DFS). The logged (ln) hazard ratio (HR) was calculated and used as the statistic of interest. Data was grouped by follow-up.
Results: Meta-analyses found no significant difference in OS at 5, 10 and 25-years follow-up (5-year ln HR = 0.08, 95% CI - 0.09, 0.25, 10-year ln HR = 0.33, 95% CI - 0.07, 0.72, 25-year ln HR = 0.00, 95% CI - 0.18, 0.19). ALND caused improvement in DFS at 5-years follow-up (ln HR = 0.16, 95% CI 0.03, 0.29), this was not demonstrated at 10 and 25-years follow-up (10-year ln HR = 0.07, 95% CI - 0.09, 0.23, 25-year ln HR = - 0.03, 95% CI - 0.21, 0.16). Studies supporting ALND for DFS at 5-years follow-up had greater relative chemotherapy use in the ALND cohort.
Conclusion: ALND does not cause a significant improvement in OS in women with clinically node-negative breast cancer. ALND may improve DFS in the short term by tailoring a proportion of patients towards chemotherapy. Our evidence suggests that when the administration of systemic therapy is balanced between the two arms, axillary de-escalation studies will likely find no difference in OS or DFS.
Keywords: Axillary clearance; Axillary dissection; Axillary lymph nodes; Early breast cancer.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial.JAMA. 2017 Sep 12;318(10):918-926. doi: 10.1001/jama.2017.11470. JAMA. 2017. PMID: 28898379 Free PMC article. Clinical Trial.
-
Recommendation for axillary lymph node dissection in women with early breast cancer and sentinel node metastasis: A systematic review and meta-analysis of randomized controlled trials using the GRADE system.Int J Surg. 2016 Oct;34:73-80. doi: 10.1016/j.ijsu.2016.08.022. Epub 2016 Aug 22. Int J Surg. 2016. PMID: 27562691 Review.
-
Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial.Lancet Oncol. 2010 Oct;11(10):927-33. doi: 10.1016/S1470-2045(10)70207-2. Lancet Oncol. 2010. PMID: 20863759 Free PMC article. Clinical Trial.
-
Comparing Observation, Axillary Radiotherapy, and Completion Axillary Lymph Node Dissection for Management of Axilla in Breast Cancer in Patients with Positive Sentinel Nodes: A Systematic Review.Ann Surg Oncol. 2020 Aug;27(8):2664-2676. doi: 10.1245/s10434-020-08225-y. Epub 2020 Feb 4. Ann Surg Oncol. 2020. PMID: 32020394
-
Impact of axillary lymph node dissection on breast cancer outcome in clinically node negative patients: a systematic review and meta-analysis.Cancer. 2009 Apr 15;115(8):1613-20. doi: 10.1002/cncr.24174. Cancer. 2009. PMID: 19199349 Review.
References
-
- Giuliano A, Ballman K, McCall L, Beitsch P, Morrow M. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis. JAMA - J Am Med Assoc. 2017;318(10):918–926. doi: 10.1001/jama.2017.11470. - DOI - PMC - PubMed
-
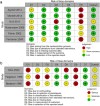
- J. P. Higgins, J. Savović, M. J. Page, R. G. Elbers, and J. A. Sterne, “Assessing risk of bias in a randomized trial,” in Cochrane Handbook for Systematic Reviews of Interventions, 6.1., J. Higgins, J. Savović, P. MJ, E. RG, and S. JAC, Eds. Cochrane, 2019, pp. 205–228.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

