Comprehensive Analysis of Peripheral Exosomal circRNAs in Large Artery Atherosclerotic Stroke
- PMID: 34239876
- PMCID: PMC8257506
- DOI: 10.3389/fcell.2021.685741
Comprehensive Analysis of Peripheral Exosomal circRNAs in Large Artery Atherosclerotic Stroke
Abstract
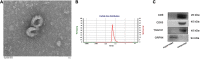
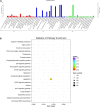
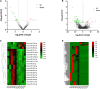
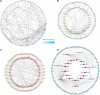
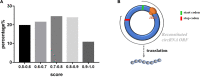
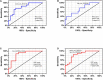
Exosomes are crucial vehicles in intercellular communication. Circular RNAs (circRNAs), novel endogenous noncoding RNAs, play diverse roles in ischemic stroke. Recently, the abundance and stability of circRNAs in exosomes have been identified. However, a comprehensive analysis of exosomal circRNAs in large artery atherosclerotic (LAA) stroke has not yet been reported. We performed RNA sequencing (RNA-Seq) to comprehensively identify differentially expressed (DE) exosomal circRNAs in five paired LAA and normal controls. Further, quantitative real-time PCR (qRT-PCR) was used to verify the RNA-Seq results in a cohort of stroke patients (32 versus 32). RNA-Seq identified a total of 462 circRNAs in peripheral exosomes; there were 25 DE circRNAs among them. Additionally, circRNA competing endogenous RNA (ceRNA) network and translatable analysis revealed the potential functions of the exosomal circRNAs in LAA progression. Two ceRNA pathways involving 5 circRNAs, 2 miRNAs, and 3 mRNAs were confirmed by qRT-PCR. In the validation cohort, receiver operating characteristic (ROC) curve analysis identified two circRNAs as possible novel biomarkers, and a logistic model combining two and four circRNAs increased the area under the curve compared with the individual circRNAs. Here, we show for the first time the comprehensive expression of exosomal circRNAs, which displayed the potential diagnostic and biological function in LAA stroke.
Keywords: RNA sequencing; ceRNA network; circular RNAs; exosomes; large artery atherosclerotic stroke.
Copyright © 2021 Xiao, Yin, Wang, Yang, Ma, Pan and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Circulating Exosomal circRNAs Contribute to Potential Diagnostic Value of Large Artery Atherosclerotic Stroke.Front Immunol. 2022 Jan 13;12:830018. doi: 10.3389/fimmu.2021.830018. eCollection 2021. Front Immunol. 2022. PMID: 35095932 Free PMC article.
-
Identification of Circular RNA hsa_circ_0001599 as a Novel Biomarker for Large-Artery Atherosclerotic Stroke.DNA Cell Biol. 2021 Mar;40(3):457-468. doi: 10.1089/dna.2020.5662. Epub 2021 Jan 25. DNA Cell Biol. 2021. PMID: 33493415 Clinical Trial.
-
Construction of circRNA-Mediated Immune-Related ceRNA Network and Identification of Circulating circRNAs as Diagnostic Biomarkers in Acute Ischemic Stroke.J Inflamm Res. 2022 Jul 17;15:4087-4104. doi: 10.2147/JIR.S368417. eCollection 2022. J Inflamm Res. 2022. PMID: 35873383 Free PMC article.
-
Exosomal circRNAs: biogenesis, effect and application in human diseases.Mol Cancer. 2019 Jul 5;18(1):116. doi: 10.1186/s12943-019-1041-z. Mol Cancer. 2019. PMID: 31277663 Free PMC article. Review.
-
Emerging Roles of Exosomal Circular RNAs in Cancer.Front Cell Dev Biol. 2020 Oct 8;8:568366. doi: 10.3389/fcell.2020.568366. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33117799 Free PMC article. Review.
Cited by
-
A Novel Perspective on Ischemic Stroke: A Review of Exosome and Noncoding RNA Studies.Brain Sci. 2022 Jul 28;12(8):1000. doi: 10.3390/brainsci12081000. Brain Sci. 2022. PMID: 36009062 Free PMC article. Review.
-
A case-control comparison of acute-phase peripheral blood gene expression in participants diagnosed with minor ischaemic stroke or stroke mimics.Hum Genomics. 2023 Nov 25;17(1):106. doi: 10.1186/s40246-023-00551-y. Hum Genomics. 2023. PMID: 38007520 Free PMC article.
-
Impact of CircRNAs on Ischemic Stroke.Aging Dis. 2022 Apr 1;13(2):329-339. doi: 10.14336/AD.2021.1113. eCollection 2022 Apr. Aging Dis. 2022. PMID: 35371609 Free PMC article.
-
Circular RNA as biomarkers for acute ischemic stroke: A systematic review and meta-analysis.CNS Neurosci Ther. 2023 Aug;29(8):2086-2100. doi: 10.1111/cns.14220. Epub 2023 Apr 26. CNS Neurosci Ther. 2023. PMID: 37186176 Free PMC article. Review.
-
Profile and Functional Prediction of Plasma Exosome-Derived CircRNAs From Acute Ischemic Stroke Patients.Front Genet. 2022 Mar 14;13:810974. doi: 10.3389/fgene.2022.810974. eCollection 2022. Front Genet. 2022. PMID: 35360855 Free PMC article.
References
-
- Adams H. P., Jr., Bendixen B. H., Kappelle L. J., Biller J., Love B. B., Gordon D. L., et al. (1993). Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 24 35–41. 10.1161/01.str.24.1.35 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

