How Do Uterine Natural Killer and Innate Lymphoid Cells Contribute to Successful Pregnancy?
- PMID: 34234770
- PMCID: PMC8256162
- DOI: 10.3389/fimmu.2021.607669
How Do Uterine Natural Killer and Innate Lymphoid Cells Contribute to Successful Pregnancy?
Abstract
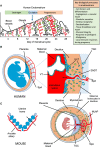
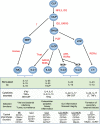
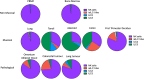
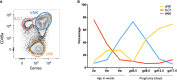
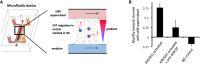
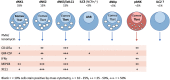
Innate lymphoid cells (ILCs) are the most abundant immune cells in the uterine mucosa both before and during pregnancy. Circumstantial evidence suggests they play important roles in regulating placental development but exactly how they contribute to the successful outcome of pregnancy is still unclear. Uterine ILCs (uILCs) include subsets of tissue-resident natural killer (NK) cells and ILCs, and until recently the phenotype and functions of uILCs were poorly defined. Determining the specific roles of each subset is intrinsically challenging because of the rapidly changing nature of the tissue both during the menstrual cycle and pregnancy. Single-cell RNA sequencing (scRNAseq) and high dimensional flow and mass cytometry approaches have recently been used to analyse uILC populations in the uterus in both humans and mice. This detailed characterisation has significantly changed our understanding of the heterogeneity within the uILC compartment. It will also enable key clinical questions to be addressed including whether specific uILC subsets are altered in infertility, miscarriage and pregnancy disorders such as foetal growth restriction and pre-eclampsia. Here, we summarise recent advances in our understanding of the phenotypic and functional diversity of uILCs in non-pregnant endometrium and first trimester decidua, and review how these cells may contribute to successful placental development.
Keywords: decidua; endometrium; innate lymphoid cell; placenta; pregnancy; tissue resident natural killer cell; uterine natural killer cell.
Copyright © 2021 Huhn, Zhao, Esposito, Moffett, Colucci and Sharkey.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Uterine Natural Killer Cells.Front Immunol. 2019 May 1;10:960. doi: 10.3389/fimmu.2019.00960. eCollection 2019. Front Immunol. 2019. PMID: 31118936 Free PMC article. Review.
-
Composition, Development, and Function of Uterine Innate Lymphoid Cells.J Immunol. 2015 Oct 15;195(8):3937-45. doi: 10.4049/jimmunol.1500689. Epub 2015 Sep 14. J Immunol. 2015. PMID: 26371244 Free PMC article.
-
Heterogeneity of NK Cells and Other Innate Lymphoid Cells in Human and Murine Decidua.Front Immunol. 2019 Feb 8;10:170. doi: 10.3389/fimmu.2019.00170. eCollection 2019. Front Immunol. 2019. PMID: 30800126 Free PMC article. Review.
-
Molecular characteristics and possible functions of innate lymphoid cells in the uterus and gut.Cytokine Growth Factor Rev. 2020 Apr;52:15-24. doi: 10.1016/j.cytogfr.2019.11.003. Epub 2019 Nov 15. Cytokine Growth Factor Rev. 2020. PMID: 31771906 Review.
-
Dynamic Changes in Uterine NK Cell Subset Frequency and Function Over the Menstrual Cycle and Pregnancy.Front Immunol. 2022 Jun 16;13:880438. doi: 10.3389/fimmu.2022.880438. eCollection 2022. Front Immunol. 2022. PMID: 35784314 Free PMC article.
Cited by
-
Local immune recognition of trophoblast in early human pregnancy: controversies and questions.Nat Rev Immunol. 2023 Apr;23(4):222-235. doi: 10.1038/s41577-022-00777-2. Epub 2022 Oct 3. Nat Rev Immunol. 2023. PMID: 36192648 Free PMC article. Review.
-
Deciphering the Epigenetic Landscape: Placental Development and Its Role in Pregnancy Outcomes.Stem Cell Rev Rep. 2024 May;20(4):996-1014. doi: 10.1007/s12015-024-10699-2. Epub 2024 Mar 8. Stem Cell Rev Rep. 2024. PMID: 38457061 Review.
-
Maternal obesity and the impact of associated early-life inflammation on long-term health of offspring.Front Cell Infect Microbiol. 2022 Sep 16;12:940937. doi: 10.3389/fcimb.2022.940937. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36189369 Free PMC article. Review.
-
Uterine NK Cells Ace an "A" in Education: NKG2A Sets Up Crucial Functions at the Maternal-Fetal Interface.J Immunol. 2022 Oct 15;209(8):1421-1425. doi: 10.4049/jimmunol.2200384. J Immunol. 2022. PMID: 36192118 Free PMC article. Review.
-
Number and function of uterine natural killer cells in recurrent miscarriage and implantation failure: a systematic review and meta-analysis.Hum Reprod Update. 2022 Jun 30;28(4):548-582. doi: 10.1093/humupd/dmac006. Hum Reprod Update. 2022. PMID: 35265977 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

