Evidence-based clinical practice guidelines for Liver Cirrhosis 2020
- PMID: 34231046
- PMCID: PMC8280040
- DOI: 10.1007/s00535-021-01788-x
Evidence-based clinical practice guidelines for Liver Cirrhosis 2020
Abstract
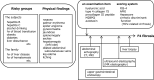
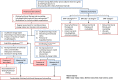
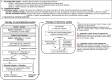
The first edition of the clinical practice guidelines for liver cirrhosis was published in 2010, and the second edition was published in 2015 by the Japanese Society of Gastroenterology (JSGE). The revised third edition was recently published in 2020. This version has become a joint guideline by the JSGE and the Japan Society of Hepatology (JSH). In addition to the clinical questions (CQs), background questions (BQs) are new items for basic clinical knowledge, and future research questions (FRQs) are newly added clinically important items. Concerning the clinical treatment of liver cirrhosis, new findings have been reported over the past 5 years since the second edition. In this revision, we decided to match the international standards as much as possible by referring to the latest international guidelines. Newly developed agents for various complications have also made great progress. In comparison with the latest global guidelines, such as the European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD), we are introducing data based on the evidence for clinical practice in Japan. The flowchart for nutrition therapy was reviewed to be useful for daily medical care by referring to overseas guidelines. We also explain several clinically important items that have recently received focus and were not mentioned in the last editions. This digest version describes the issues related to the management of liver cirrhosis and several complications in clinical practice. The content begins with a diagnostic algorithm, the revised flowchart for nutritional therapy, and refracted ascites, which are of great importance to patients with cirrhosis. In addition to the updated antiviral therapy for hepatitis B and C liver cirrhosis, the latest treatments for non-viral cirrhosis, such as alcoholic steatohepatitis/non-alcoholic steatohepatitis (ASH/NASH) and autoimmune-related cirrhosis, are also described. It also covers the latest evidence regarding the diagnosis and treatment of liver cirrhosis complications, namely gastrointestinal bleeding, ascites, hepatorenal syndrome and acute kidney injury, hepatic encephalopathy, portal thrombus, sarcopenia, muscle cramp, thrombocytopenia, pruritus, hepatopulmonary syndrome, portopulmonary hypertension, and vitamin D deficiency, including BQ, CQ and FRQ. Finally, this guideline covers prognosis prediction and liver transplantation, especially focusing on several new findings since the last version. Since this revision is a joint guideline by both societies, the same content is published simultaneously in the official English journal of JSGE and JSH.
Keywords: Complications; Diagnosis; Guidelines; Liver cirrhosis; Treatment.
© 2021. The Author(s).
Conflict of interest statement
Any financial relationship with enterprises, businesses or academic institutions in the subject matter or materials discussed in the manuscript are listed as follows: (1) those from which the enterprises and for-profit organizations received remuneration paid as patent royalties; SRL, (2) those from which the authors, the spouse, partner or immediate relatives of the authors have received individually any income, honoraria or any other type of renumeration; Abbvie, Otsuka Pharmaceutical, Gilead Sciences, Sumitomo Dainippon Pharma, MSD, Eisai, EA Pharma, Bristol Myers Squibb, Mitsubishi Tanabe Pharma, Bayer Yakuhin, ASKA Pharmaceutical, Daiichi Sankyo, Takeda Pharmaceutical, Taiho Pharmaceutical, Chugai Pharmaceutical, (3) those from which the academic institutions of the authors received support (commercial/academic cooperation); Abbvie, Otsuka Pharmaceutical, EA Pharma, Gilead Sciences, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Bristol Myers Squibb, Eisai, MSD, Nippon Kayaku, Ono Pharmaceutical, Teijin Pharma, Novartis Pharma, ASKA Pharmaceutical, Astellas Pharmaceutical, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Chugai Pharmaceutical, Toray, Mochida Pharmaceutical, Interstem, Rohto Pharmaceutical, Towa Pharmaceutical, Nihon Pharmaceutical, Janssen Pharmaceutical, MIC Medical, Kowa, and 4)those from which the authors have received individually endowed chair;Shibuya Corporation.
Figures

Similar articles
-
Evidence-based clinical practice guidelines for liver cirrhosis 2020.Hepatol Res. 2021 Jul;51(7):725-749. doi: 10.1111/hepr.13678. Epub 2021 Jul 6. Hepatol Res. 2021. PMID: 34228859
-
Evidence-based clinical practice guidelines for liver cirrhosis 2015.J Gastroenterol. 2016 Jul;51(7):629-50. doi: 10.1007/s00535-016-1216-y. Epub 2016 May 31. J Gastroenterol. 2016. PMID: 27246107 Review.
-
Usefulness of nutritional therapy recommended in the Japanese Society of Gastroenterology/Japan Society of Hepatology evidence-based clinical practice guidelines for liver cirrhosis 2020.J Gastroenterol. 2021 Oct;56(10):928-937. doi: 10.1007/s00535-021-01821-z. Epub 2021 Sep 17. J Gastroenterol. 2021. PMID: 34533633
-
Evidence-based clinical practice guidelines for cholelithiasis 2021.J Gastroenterol. 2023 Sep;58(9):801-833. doi: 10.1007/s00535-023-02014-6. Epub 2023 Jul 15. J Gastroenterol. 2023. PMID: 37452855 Free PMC article. Review.
-
Evidence-based clinical practice guidelines for management of colorectal polyps.J Gastroenterol. 2021 Apr;56(4):323-335. doi: 10.1007/s00535-021-01776-1. Epub 2021 Mar 12. J Gastroenterol. 2021. PMID: 33710392 Free PMC article. Review.
Cited by
-
Effectiveness of antibiotic prophylaxis for acute esophageal variceal bleeding in patients with band ligation: A large observational study.World J Gastroenterol. 2024 Jan 21;30(3):238-251. doi: 10.3748/wjg.v30.i3.238. World J Gastroenterol. 2024. PMID: 38314133 Free PMC article.
-
Prognostic Factors for Overall Survival in Patients with HCV-Related HCC Undergoing Molecular Targeted Therapies: Beyond a Sustained Virological Response.Cancers (Basel). 2022 Oct 4;14(19):4850. doi: 10.3390/cancers14194850. Cancers (Basel). 2022. PMID: 36230773 Free PMC article.
-
VariScreen secures the screening of high-risk varices in patients with hepatitis B virus-related cirrhosis beyond Baveno VI criteria.Front Physiol. 2022 Sep 27;13:1006657. doi: 10.3389/fphys.2022.1006657. eCollection 2022. Front Physiol. 2022. PMID: 36237519 Free PMC article.
-
Deep learning-based 3D quantitative total tumor burden predicts early recurrence of BCLC A and B HCC after resection.Eur Radiol. 2025 Jan;35(1):127-139. doi: 10.1007/s00330-024-10941-y. Epub 2024 Jul 19. Eur Radiol. 2025. PMID: 39028376 Free PMC article.
-
Endotoxin Activity Reflects an Increase in Body Temperature in Cirrhotic Patients With Ascites Undergoing Cell-free and Concentrated Ascites Reinfusion Therapy.In Vivo. 2022 May-Jun;36(3):1477-1484. doi: 10.21873/invivo.12854. In Vivo. 2022. PMID: 35478114 Free PMC article.
References
-
- Higgins JPT, Thomas J, Chandler J. Cochrane Handbook for Systematic Reviews of Interventions version 6.0. https://training.cochrane.org/handbook/current. 2019.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

