Clathrin Is Important for Virulence Factors Delivery in the Necrotrophic Fungus Botrytis cinerea
- PMID: 34220891
- PMCID: PMC8244658
- DOI: 10.3389/fpls.2021.668937
Clathrin Is Important for Virulence Factors Delivery in the Necrotrophic Fungus Botrytis cinerea
Abstract
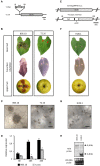
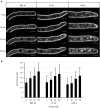
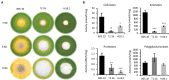
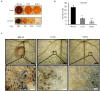
Fungi are the most prevalent plant pathogens, causing annually important damages. To infect and colonize their hosts, they secrete effectors including hydrolytic enzymes able to kill and macerate plant tissues. These secreted proteins are transported from the Endoplasmic Reticulum and the Golgi apparatus to the extracellular space through intracellular vesicles. In pathogenic fungi, intracellular vesicles were described but their biogenesis and their role in virulence remain unclear. In this study, we report the essential role of clathrin heavy chain (CHC) in the pathogenicity of Botrytis cinerea, the agent of gray mold disease. To investigate the importance of this protein involved in coat vesicles formation in eukaryotic cells, a T-DNA insertional mutant reduced in the expression of the CHC-encoding gene, and a mutant expressing a dominant-negative form of CHC were studied. Both mutants were strongly affected in pathogenicity. Characterization of the mutants revealed altered infection cushions and an important defect in protein secretion. This study demonstrates the essential role of clathrin in the infectious process of a plant pathogenic fungus and more particularly its role in virulence factors delivery.
Keywords: Botrytis cinerea; clathrin; infection cushion; secretomics; virulence.
Copyright © 2021 Souibgui, Bruel, Choquer, de Vallée, Dieryckx, Dupuy, Latorse, Rascle and Poussereau.
Conflict of interest statement
M-PL was employed by the company Bayer SAS. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The GATA transcription factor BcWCL2 regulates citric acid secretion to maintain redox homeostasis and full virulence in Botrytis cinerea.mBio. 2024 Jul 17;15(7):e0013324. doi: 10.1128/mbio.00133-24. Epub 2024 May 30. mBio. 2024. PMID: 38814088 Free PMC article.
-
A Similar Secretome Disturbance as a Hallmark of Non-pathogenic Botrytis cinerea ATMT-Mutants?Front Microbiol. 2019 Dec 6;10:2829. doi: 10.3389/fmicb.2019.02829. eCollection 2019. Front Microbiol. 2019. PMID: 31866989 Free PMC article.
-
Role of BcSfb3, the subunit of COPII vesicles, in fungal development and pathogenicity, ER-phagy and autophagy in the gray mold fungus Botrytis cinerea.Int J Biol Macromol. 2024 Apr;263(Pt 2):130379. doi: 10.1016/j.ijbiomac.2024.130379. Epub 2024 Feb 24. Int J Biol Macromol. 2024. PMID: 38403214
-
Unraveling the in vitro secretome of the phytopathogen Botrytis cinerea to understand the interaction with its hosts.Front Plant Sci. 2015 Oct 9;6:839. doi: 10.3389/fpls.2015.00839. eCollection 2015. Front Plant Sci. 2015. PMID: 26500673 Free PMC article. Review.
-
Botrytis cinerea virulence factors: new insights into a necrotrophic and polyphageous pathogen.FEMS Microbiol Lett. 2007 Dec;277(1):1-10. doi: 10.1111/j.1574-6968.2007.00930.x. FEMS Microbiol Lett. 2007. PMID: 17986079 Review.
Cited by
-
A fungal phospholipase C involved in the degradation of plant glycosylinositol phosphorylceramides during Arabidopsis/Botrytis interaction.Commun Biol. 2024 Oct 22;7(1):1372. doi: 10.1038/s42003-024-07064-x. Commun Biol. 2024. PMID: 39438581 Free PMC article.
-
BAR domain is essential for early endosomal trafficking and dynamics in Ascochyta rabiei.3 Biotech. 2023 Feb;13(2):49. doi: 10.1007/s13205-022-03451-5. Epub 2023 Jan 17. 3 Biotech. 2023. PMID: 36685317 Free PMC article.
-
Snf1 Kinase Differentially Regulates Botrytis cinerea Pathogenicity according to the Plant Host.Microorganisms. 2022 Feb 15;10(2):444. doi: 10.3390/microorganisms10020444. Microorganisms. 2022. PMID: 35208900 Free PMC article.
-
Editorial: Highlights from the Botrytis and Sclerotinia 2022 Joint Conference.Front Plant Sci. 2023 Nov 8;14:1326020. doi: 10.3389/fpls.2023.1326020. eCollection 2023. Front Plant Sci. 2023. PMID: 38023886 Free PMC article. No abstract available.
-
Cell-Wall-Degrading Enzymes-Related Genes Originating from Rhizoctonia solani Increase Sugar Beet Root Damage in the Presence of Leuconostoc mesenteroides.Int J Mol Sci. 2022 Jan 25;23(3):1366. doi: 10.3390/ijms23031366. Int J Mol Sci. 2022. PMID: 35163289 Free PMC article.
References
LinkOut - more resources
Full Text Sources

