Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: a first-in-human, phase 1, dose-escalation trial
- PMID: 34214495
- PMCID: PMC8328944
- DOI: 10.1016/S1470-2045(21)00245-X
Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: a first-in-human, phase 1, dose-escalation trial
Abstract
Background: Malignant glioma is the most common and lethal primary brain tumour, with dismal survival rates and no effective treatment. We examined the safety and activity of NSC-CRAd-S-pk7, an engineered oncolytic adenovirus delivered by neural stem cells (NSCs), in patients with newly diagnosed high-grade glioma.
Methods: This was a first-in-human, open-label, phase 1, dose-escalation trial done to determine the maximal tolerated dose of NSC-CRAd-S-pk7, following a 3 + 3 design. Patients with newly diagnosed, histologically confirmed, high-grade gliomas (WHO grade III or IV) were recruited. After neurosurgical resection, NSC-CRAd-S-pk7 was injected into the walls of the resection cavity. The first patient cohort received a dose starting at 6·25 × 1010 viral particles administered by 5·00 × 107 NSCs, the second cohort a dose of 1·25 × 1011 viral particles administered by 1·00 × 108 NSCs, and the third cohort a dose of 1·875 × 1011 viral particles administered by 1·50 × 108 NSCs. No further dose escalation was planned. Within 10-14 days, treatment with temozolomide and radiotherapy was initiated. Primary endpoints were safety and toxicity profile and the maximum tolerated dose for a future phase 2 trial. All analyses were done in all patients who were included in the trial and received the study treatment and were not excluded from the study. Recruitment is complete and the trial is finished. The trial is registered with ClinicalTrials.gov, NCT03072134.
Findings: Between April 24, 2017, and Nov 13, 2019, 12 patients with newly diagnosed, malignant gliomas were recruited and included in the safety analysis. Histopathological evaluation identified 11 (92%) of 12 patients with glioblastoma and one (8%) of 12 patients with anaplastic astrocytoma. The median follow-up was 18 months (IQR 14-22). One patient receiving 1·50 × 108 NSCs loading 1·875 × 1011 viral particles developed viral meningitis (grade 3) due to the inadvertent injection of NSC-CRAd-S-pk7 into the lateral ventricle. Otherwise, treatment was safe as no formal dose-limiting toxicity was reached, so 1·50 × 108 NSCs loading 1·875 × 1011 viral particles was recommended as a phase 2 trial dose. There were no treatment-related deaths. The median progression-free survival was 9·1 months (95% CI 8·5-not reached) and median overall survival was 18·4 months (15·7-not reached).
Interpretation: NSC-CRAd-S-pk7 treatment was feasible and safe. Our immunological and histopathological findings support continued investigation of NSC-CRAd-S-pk7 in a phase 2/3 clinical trial.
Funding: US National Institutes of Health.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests JP reports grants from The Ivy Foundation, during the conduct of this study. CDH reports salary payments from Southern Research, outside the submitted work. RVL reports honoraria from Novocure for advisory roles, EBSCO Publishing and Medlink Neurology for medical editing, ECRI for reviewing medical content, and the American Physician Institute for creating and presenting board review continuing medical education material, outside the submitted work. RS reports non-financial support from CarThera, and personal fees from Celularity, CranioVation, TriAct, Hemispherian, Northwest Biotherapeutics, GT Medical Technologies, Insightec, and ZaiLab, outside the submitted work. DTC, KSA, and MSL have an issued patent that is related to the study (US10238699 and US10709745). KSA was the CSO and Director of TheraBiologics (company in process of being dissolved) during the conduct of this study; she neither has assets nor receives financial benefit from the company. MSL reports grants from the National Institutes of Health, during the conduct of this study. All other authors declare no competing interests.
Figures

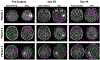
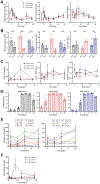

Comment in
-
Hitchhiking to brain tumours: stem cell delivery of oncolytic viruses.Lancet Oncol. 2021 Aug;22(8):1049-1051. doi: 10.1016/S1470-2045(21)00296-5. Epub 2021 Jun 29. Lancet Oncol. 2021. PMID: 34214494 No abstract available.
Similar articles
-
Pharmacokinetic study of neural stem cell-based cell carrier for oncolytic virotherapy: targeted delivery of the therapeutic payload in an orthotopic brain tumor model.Cancer Gene Ther. 2012 Jun;19(6):431-42. doi: 10.1038/cgt.2012.21. Epub 2012 May 4. Cancer Gene Ther. 2012. PMID: 22555507 Free PMC article.
-
N-acetylcysteine amide augments the therapeutic effect of neural stem cell-based antiglioma oncolytic virotherapy.Mol Ther. 2013 Nov;21(11):2063-73. doi: 10.1038/mt.2013.179. Epub 2013 Jul 25. Mol Ther. 2013. PMID: 23883863 Free PMC article.
-
The timing of neural stem cell-based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma.Stem Cells Transl Med. 2013 Sep;2(9):655-66. doi: 10.5966/sctm.2013-0039. Epub 2013 Aug 7. Stem Cells Transl Med. 2013. PMID: 23926209 Free PMC article.
-
Maintaining and loading neural stem cells for delivery of oncolytic adenovirus to brain tumors.Methods Mol Biol. 2012;797:97-109. doi: 10.1007/978-1-61779-340-0_8. Methods Mol Biol. 2012. PMID: 21948472 Review.
-
Oncolytic Virotherapy for the Treatment of Malignant Glioma.Neurotherapeutics. 2017 Apr;14(2):333-344. doi: 10.1007/s13311-017-0516-0. Neurotherapeutics. 2017. PMID: 28265902 Free PMC article. Review.
Cited by
-
Joining Forces: The Combined Application of Therapeutic Viruses and Nanomaterials in Cancer Therapy.Molecules. 2023 Nov 20;28(22):7679. doi: 10.3390/molecules28227679. Molecules. 2023. PMID: 38005401 Free PMC article. Review.
-
Breaking Barriers: A Future Perspective on Glioblastoma Therapy with mRNA-Based Immunotherapies and Oncolytic Viruses.Vaccines (Basel). 2024 Jan 8;12(1):61. doi: 10.3390/vaccines12010061. Vaccines (Basel). 2024. PMID: 38250874 Free PMC article.
-
Oncolytic Virotherapy: From Bench to Bedside.Front Cell Dev Biol. 2021 Nov 26;9:790150. doi: 10.3389/fcell.2021.790150. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34901031 Free PMC article. Review.
-
An Overview of Advances in Rare Cancer Diagnosis and Treatment.Int J Mol Sci. 2024 Jan 18;25(2):1201. doi: 10.3390/ijms25021201. Int J Mol Sci. 2024. PMID: 38256274 Free PMC article. Review.
-
Remission of liquid tumors and SARS-CoV-2 infection: A literature review.Mol Ther Oncolytics. 2022 Sep 15;26:135-140. doi: 10.1016/j.omto.2022.06.006. Epub 2022 Jun 10. Mol Ther Oncolytics. 2022. PMID: 35702422 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352(10): 987–96. - PubMed
-
- Nicholas MK, Lukas RV, Chmura S, Yamini B, Lesniak M, Pytel P. Molecular heterogeneity in glioblastoma: therapeutic opportunities and challenges. Semin Oncol 2011; 38(2): 243–53. - PubMed
-
- Wick W, Gorlia T, Bendszus M, et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N Engl J Med 2017; 377(20): 1954–63. - PubMed
-
- Wann A, Tully PA, Barnes EH, et al. Outcomes after second surgery for recurrent glioblastoma: a retrospective case-control study. J Neurooncol 2018; 137(2): 409–15. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

