Comparison of Effectiveness Using Different Dual Bronchodilator Agents in Chronic Obstructive Pulmonary Disease Treatment
- PMID: 34208599
- PMCID: PMC8235085
- DOI: 10.3390/jcm10122649
Comparison of Effectiveness Using Different Dual Bronchodilator Agents in Chronic Obstructive Pulmonary Disease Treatment
Abstract
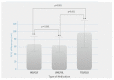
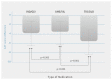
The effectiveness and safety of fixed dual long-acting bronchodilators for chronic obstructive pulmonary disease (COPD) patients have been well established; however, there is a paucity of clinical effectiveness comparison in patients with COPD treatment. The aim of the current study was to compare the effectiveness of three once-daily dual bronchodilator agents in patients with COPD. Patients with diagnosed COPD and treated with a long-acting beta-agonist (LABA) + long-acting muscarinic antagonist (LAMA) fixed-dose combination therapy (UME/VIL (umeclidinium and vilanterol inhalation powder), IND/GLY (indacaterol and glycopyrronium), and TIO/OLO (tiotropium and olodaterol)) were enrolled in this retrospective study over a period of 12 months. Effectiveness assessments were evaluated using a COPD assessment test (CAT) and lung function parameters. Besides, times for acute exacerbation were also assessed. The enrolled patients' number was 177 in IND/GLY, 176 in UME/VIL and 183 in TIO/OLO. Lung function measurements with FEV1 had significantly improved for patients using TIO/OLO (98.7 mL) compared to those of IND/GLY (65.2 mL) and UME/VIL (64.4 mL) (p < 0.001). CAT scores were also significantly decreased in patients treated with TIO/OLO (CAT down 5.6) than those with IND/GLY (3.8) and UME/VIL (3.9) (p = 0.03). Acute exacerbation was also reduced in patients using TIO/OLO (4.9%) compared with those using IND/GLY (10.2%) and UME/VIL (11.9%) (p = 0.01). Significant improvement in pulmonary function, symptoms were demonstrated after 12 months of LABA/LAMA fixed-dose combination therapy with three different treatment options. TIO/OLO demonstrated higher therapeutic effects compared with UME/VIL or IND/GLY. Determining clinical relevance will require a well-designed randomized controlled trial.
Keywords: LABA/LAMA; chronic obstructive pulmonary disease (COPD); dual bronchodilator.
Conflict of interest statement
The authors declare that they have no competing interests. The manuscript reports studies involving human participants, human data, and statement on ethics approval and consent is IBR Number: 107006-E, and the name of the ethics committee is Research Ethics Review Committee, Far Eastern Memorial Hospital.
Figures

Similar articles
-
Differences in Pulmonary Function Improvement after Once-Daily LABA/LAMA Fixed-Dose Combinations in Patients with COPD.J Clin Med. 2022 Dec 1;11(23):7165. doi: 10.3390/jcm11237165. J Clin Med. 2022. PMID: 36498738 Free PMC article.
-
Real-world comparative effectiveness of three single-inhaler dual bronchodilators for the treatment of COPD.Eur Respir J. 2023 Aug 3;62(2):2300538. doi: 10.1183/13993003.00538-2023. Print 2023 Aug. Eur Respir J. 2023. PMID: 37343975
-
A randomized controlled trial of long-acting muscarinic antagonist and long-acting β2 agonist fixed-dose combinations in patients with chronic obstructive pulmonary disease.BMC Pulm Med. 2021 Jan 13;21(1):26. doi: 10.1186/s12890-021-01403-y. BMC Pulm Med. 2021. PMID: 33441146 Free PMC article. Clinical Trial.
-
Pharmacoeconomic Review Report: Tiotropium bromide monohydrate and olodaterol hydrochloride (Inspiolto Respimat) for oral inhalation [Internet].Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2015 Dec. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2015 Dec. PMID: 30883067 Free Books & Documents. Review.
-
Efficacy and safety of tiotropium and olodaterol in COPD: a systematic review and meta-analysis.Respir Res. 2017 Nov 25;18(1):196. doi: 10.1186/s12931-017-0683-x. Respir Res. 2017. PMID: 29178871 Free PMC article. Review.
Cited by
-
Clinical Implications of Peak Inspiratory Flow in COPD: Post Hoc Analyses of the TRONARTO Study.Int J Chron Obstruct Pulmon Dis. 2023 Aug 10;18:1729-1740. doi: 10.2147/COPD.S404243. eCollection 2023. Int J Chron Obstruct Pulmon Dis. 2023. PMID: 37599896 Free PMC article.
-
Differences in Pulmonary Function Improvement after Once-Daily LABA/LAMA Fixed-Dose Combinations in Patients with COPD.J Clin Med. 2022 Dec 1;11(23):7165. doi: 10.3390/jcm11237165. J Clin Med. 2022. PMID: 36498738 Free PMC article.
-
Shared Decision-Making Facilitates Inhaler Choice in Patients with Newly-Diagnosed Chronic Obstructive Pulmonary Disease: A Multicenter Prospective Study.Int J Chron Obstruct Pulmon Dis. 2022 Sep 2;17:2067-2078. doi: 10.2147/COPD.S376547. eCollection 2022. Int J Chron Obstruct Pulmon Dis. 2022. PMID: 36081765 Free PMC article.
References
-
- Ko F.W.S., Hui D., Lai C.K.W. Worldwide burden of COPD in high- and low-income countries. Part III. Asia-Pacific studies. Int. J. Tuberc. Lung Dis. 2008;12:713–717. - PubMed
-
- Lim S., Lam D.C.-L., Muttalif A.R., Yunus F., Wongtim S., Lan L.T.T., Shetty V., Chu R., Zheng J., Perng D.-W., et al. Impact of chronic obstructive pulmonary disease (COPD) in the Asia-Pacific region: The EPIC Asia population-based survey. Asia Pac. Fam. Med. 2015;14:4. doi: 10.1186/s12930-015-0020-9. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

