The Journey of DDR1 and DDR2 Kinase Inhibitors as Rising Stars in the Fight Against Cancer
- PMID: 34207360
- PMCID: PMC8235339
- DOI: 10.3390/ijms22126535
The Journey of DDR1 and DDR2 Kinase Inhibitors as Rising Stars in the Fight Against Cancer
Abstract
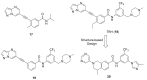
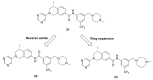
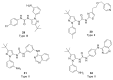
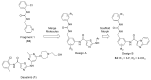
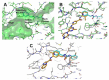
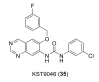
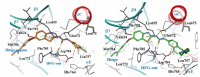
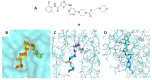
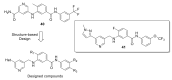
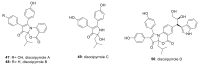
Discoidin domain receptor (DDR) is a collagen-activated receptor tyrosine kinase that plays critical roles in regulating essential cellular processes such as morphogenesis, differentiation, proliferation, adhesion, migration, invasion, and matrix remodeling. As a result, DDR dysregulation has been attributed to a variety of human cancer disorders, for instance, non-small-cell lung carcinoma (NSCLC), ovarian cancer, glioblastoma, and breast cancer, in addition to some inflammatory and neurodegenerative disorders. Since the target identification in the early 1990s to date, a lot of efforts have been devoted to the development of DDR inhibitors. From a medicinal chemistry perspective, we attempted to reveal the progress in the development of the most promising DDR1 and DDR2 small molecule inhibitors covering their design approaches, structure-activity relationship (SAR), biological activity, and selectivity.
Keywords: DDR1 and DDR2; cancer; discoidin domain receptor (DDR); kinase inhibitors; structure-activity relationship (SAR).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

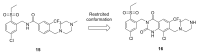

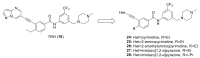




Similar articles
-
Inhibitors of Discoidin Domain Receptor (DDR) Kinases for Cancer and Inflammation.Biomolecules. 2021 Nov 10;11(11):1671. doi: 10.3390/biom11111671. Biomolecules. 2021. PMID: 34827669 Free PMC article. Review.
-
Synthesis and biological evaluation of novel dasatinib analogues as potent DDR1 and DDR2 kinase inhibitors.Chem Biol Drug Des. 2017 Mar;89(3):420-427. doi: 10.1111/cbdd.12863. Epub 2016 Oct 17. Chem Biol Drug Des. 2017. PMID: 27589335
-
A patent review of discoidin domain receptor 1 (DDR1) modulators (2014-present).Expert Opin Ther Pat. 2020 May;30(5):341-350. doi: 10.1080/13543776.2020.1732925. Epub 2020 Feb 26. Expert Opin Ther Pat. 2020. PMID: 32077340 Review.
-
DDR1 and DDR2 physical interaction leads to signaling interconnection but with possible distinct functions.Cell Adh Migr. 2018;12(4):324-334. doi: 10.1080/19336918.2018.1460012. Epub 2018 Jun 25. Cell Adh Migr. 2018. PMID: 29616590 Free PMC article.
-
Identification of novel discoidin domain receptor 1 (DDR1) inhibitors using E-pharmacophore modeling, structure-based virtual screening, molecular dynamics simulation and MM-GBSA approaches.Comput Biol Med. 2022 Mar;142:105217. doi: 10.1016/j.compbiomed.2022.105217. Epub 2022 Jan 6. Comput Biol Med. 2022. PMID: 35032738
Cited by
-
A highly selective humanized DDR1 mAb reverses immune exclusion by disrupting collagen fiber alignment in breast cancer.J Immunother Cancer. 2023 Jun;11(6):e006720. doi: 10.1136/jitc-2023-006720. J Immunother Cancer. 2023. PMID: 37328286 Free PMC article.
-
Landscape of targeted therapies for lung squamous cell carcinoma.Front Oncol. 2024 Oct 31;14:1467898. doi: 10.3389/fonc.2024.1467898. eCollection 2024. Front Oncol. 2024. PMID: 39544292 Free PMC article. Review.
-
Investigation of Cell Mechanics and Migration on DDR2-Expressing Neuroblastoma Cell Line.Life (Basel). 2024 Oct 2;14(10):1260. doi: 10.3390/life14101260. Life (Basel). 2024. PMID: 39459560 Free PMC article.
-
Transcriptomic Analysis of Subtype-Specific Tyrosine Kinases as Triple Negative Breast Cancer Biomarkers.Cancers (Basel). 2023 Jan 7;15(2):403. doi: 10.3390/cancers15020403. Cancers (Basel). 2023. PMID: 36672350 Free PMC article.
-
Recent progress in targeted therapy for non-small cell lung cancer.Front Pharmacol. 2023 Feb 21;14:1125547. doi: 10.3389/fphar.2023.1125547. eCollection 2023. Front Pharmacol. 2023. PMID: 36909198 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

