Fe65: A Scaffolding Protein of Actin Regulators
- PMID: 34202290
- PMCID: PMC8304848
- DOI: 10.3390/cells10071599
Fe65: A Scaffolding Protein of Actin Regulators
Abstract
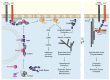
The scaffolding protein family Fe65, composed of Fe65, Fe65L1, and Fe65L2, was identified as an interaction partner of the amyloid precursor protein (APP), which plays a key function in Alzheimer's disease. All three Fe65 family members possess three highly conserved interaction domains, forming complexes with diverse binding partners that can be assigned to different cellular functions, such as transactivation of genes in the nucleus, modulation of calcium homeostasis and lipid metabolism, and regulation of the actin cytoskeleton. In this article, we rule out putative new intracellular signaling mechanisms of the APP-interacting protein Fe65 in the regulation of actin cytoskeleton dynamics in the context of various neuronal functions, such as cell migration, neurite outgrowth, and synaptic plasticity.
Keywords: Arf6; Arp2/3; DOCK; ELMO; Mena; Rac; Tip60; cortactin; neurite outgrowth; structural synaptic plasticity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
The FE65 proteins and Alzheimer's disease.J Neurosci Res. 2008 Mar;86(4):744-54. doi: 10.1002/jnr.21532. J Neurosci Res. 2008. PMID: 17828772 Review.
-
Fe65L2: a new member of the Fe65 protein family interacting with the intracellular domain of the Alzheimer's beta-amyloid precursor protein.Biochem J. 1998 Feb 15;330 ( Pt 1)(Pt 1):513-9. doi: 10.1042/bj3300513. Biochem J. 1998. PMID: 9461550 Free PMC article.
-
Fe65 is the sole member of its family that mediates transcription regulated by the amyloid precursor protein.J Cell Sci. 2020 Sep 8;133(17):jcs242917. doi: 10.1242/jcs.242917. J Cell Sci. 2020. PMID: 32843577
-
FE65 interacts with ADP-ribosylation factor 6 to promote neurite outgrowth.FASEB J. 2014 Jan;28(1):337-49. doi: 10.1096/fj.13-232694. Epub 2013 Sep 20. FASEB J. 2014. PMID: 24056087 Free PMC article.
-
FE65: Roles beyond amyloid precursor protein processing.Cell Mol Biol Lett. 2015 Mar;20(1):66-87. doi: 10.1515/cmble-2015-0002. Cell Mol Biol Lett. 2015. PMID: 26204394 Review.
Cited by
-
Age-dependent NMDA receptor function is regulated by the amyloid precursor protein.Aging Cell. 2023 Mar;22(3):e13778. doi: 10.1111/acel.13778. Epub 2023 Jan 26. Aging Cell. 2023. PMID: 36704841 Free PMC article.
-
Small GTPases control macropinocytosis of amyloid precursor protein and cleavage to amyloid-β.Heliyon. 2024 May 11;10(10):e31077. doi: 10.1016/j.heliyon.2024.e31077. eCollection 2024 May 30. Heliyon. 2024. PMID: 38799759 Free PMC article.
-
Looking at Alzheimer's Disease Pathogenesis from the Nuclear Side.Biomolecules. 2021 Aug 24;11(9):1261. doi: 10.3390/biom11091261. Biomolecules. 2021. PMID: 34572474 Free PMC article. Review.
-
Cytoskeletal dysregulation and neurodegenerative disease: Formation, monitoring, and inhibition of cofilin-actin rods.Front Cell Neurosci. 2022 Sep 22;16:982074. doi: 10.3389/fncel.2022.982074. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36212686 Free PMC article. Review.
-
Bidirectional Control between Cholesterol Shuttle and Purine Signal at the Central Nervous System.Int J Mol Sci. 2022 Aug 4;23(15):8683. doi: 10.3390/ijms23158683. Int J Mol Sci. 2022. PMID: 35955821 Free PMC article. Review.
References
-
- Bressler S.L., Gray M.D., Sopher B.L., Hu Q., Hearn M.G., Pham D.G., Dinulos M.B., Fukuchi K., Sisodia S.S., Miller M.A., et al. cDNA cloning and chromosome mapping of the human Fe65 gene: Interaction of the conserved cytoplasmic domains of the human beta-amyloid precursor protein and its homologues with the mouse Fe65 protein. Hum. Mol. Genet. 1996;5:1589–1598. doi: 10.1093/hmg/5.10.1589. - DOI - PubMed
-
- Blanco G., Irving N.G., Brown S.D., Miller C.C., McLoughlin D.M. Mapping of the human and murine X11-like genes (APBA2 and apba2), the murine Fe65 gene (Apbb1), and the human Fe65-like gene (APBB2): Genes encoding phosphotyrosine-binding domain proteins that interact with the Alzheimer’s disease amyloid precursor protein. Mamm. Genome. 1998;9:473–475. doi: 10.1007/s003359900800. - DOI - PubMed
-
- Fiore F., Zambrano N., Minopoli G., Donini V., Duilio A., Russo T. The regions of the Fe65 protein homologous to the phosphotyrosine interaction/phosphotyrosine binding domain of Shc bind the intracellular domain of the Alzheimer’s amyloid precursor protein. J. Biol. Chem. 1995;270:30853–30856. doi: 10.1074/jbc.270.52.30853. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

