Differential Modulation of 25-hydroxycholecalciferol on Innate Immunity of Broiler Breeder Hens
- PMID: 34200930
- PMCID: PMC8230489
- DOI: 10.3390/ani11061742
Differential Modulation of 25-hydroxycholecalciferol on Innate Immunity of Broiler Breeder Hens
Abstract
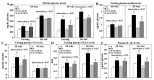
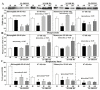
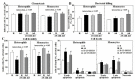
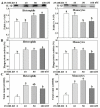
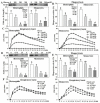
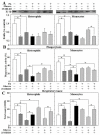
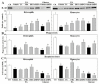
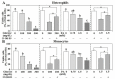
Past immunological studies in broilers focused on juveniles within the rapid pre-slaughter growth period and may not reflect adult immune responses, particularly in breeders managed with chronic feed restriction (R). The study aimed to assess innate immune cell functions in respect to R vs. ad libitum (Ad) feed intake in breeder hens with and without dietary 25-hydroxycholecalciferol (25-OH-D3) supplementation. Ad-feed intake consistently suppressed IL-1β secretion, respiratory burst, and cell livability in peripheral heterophils and/or monocytes along the feeding trial from the age of 51 to 68 weeks. Supplemental 25-OH-D3 repressed IL-1β secretion and respiratory burst of both cells mostly in R-hens, but promoted monocyte phagocytosis, chemotaxis, and bacterial killing activity in Ad-hens in accompany with relieved hyperglycemia, hyperlipidemia, and systemic inflammation. Overnight cultures with leukocytes from R-hens confirmed the differential effects of 25-OH-D3 to rescue immune functions altered by glucose and/or palmitic acid exposure. Studies with specific inhibitors further manifested the operative mechanisms via glucolipotoxicity in a cell type- and function-dependent manner. The results concluded no predominant changes between R- vs. Ad-feed intake on leukocyte defense against pathogens despite some differential differences, but supplemental 25-OH-D3 exerts more pronounced effects in Ad-hens.
Keywords: 25-hydroxycholecalciferol; broiler breeder hens; feed restriction; glucolipotoxicity; innate immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Dietary supplementation of 25-hydroxycholecalciferol improves cardiac function and livability in broiler breeder hens-amelioration of blood pressure and vascular remodeling.Poult Sci. 2020 Jul;99(7):3363-3373. doi: 10.1016/j.psj.2020.03.015. Epub 2020 Apr 17. Poult Sci. 2020. PMID: 32616230 Free PMC article.
-
Dietary supplementation of 25-hydroxycholecalciferol improves livability in broiler breeder hens.Poult Sci. 2019 Nov 1;98(11):6108-6116. doi: 10.3382/ps/pez330. Poult Sci. 2019. PMID: 31222260
-
Supplemental 25-hydroxycholecalciferol Alleviates Inflammation and Cardiac Fibrosis in Hens.Int J Mol Sci. 2020 Nov 8;21(21):8379. doi: 10.3390/ijms21218379. Int J Mol Sci. 2020. PMID: 33171670 Free PMC article.
-
Dietary Supplementation of 25-Hydroxycholecalciferol Improves Livability in Broiler Breeder Hens-Amelioration of Cardiac Pathogenesis and Hepatopathology.Animals (Basel). 2019 Oct 8;9(10):770. doi: 10.3390/ani9100770. Animals (Basel). 2019. PMID: 31597394 Free PMC article.
-
Feed restriction of turkey breeder hens--a review.Poult Sci. 1990 Sep;69(9):1439-46. doi: 10.3382/ps.0691439. Poult Sci. 1990. PMID: 2247406 Review.
Cited by
-
Preventing bacterial disease in poultry in the post-antibiotic era: a case for innate immunity modulation as an alternative to antibiotic use.Front Immunol. 2023 Jul 3;14:1205869. doi: 10.3389/fimmu.2023.1205869. eCollection 2023. Front Immunol. 2023. PMID: 37469519 Free PMC article. Review.
References
Grants and funding
- MOST 108-2321-B-005-002/Ministry of Science and Technology, Taiwan
- The iEGG and Animal Biotechnology Center and the Innovation and Development Center of Sustainable Agriculture from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project/Ministry of Education, Taiwan
- project number 8738/Texas AgriLife Research
LinkOut - more resources
Full Text Sources
Miscellaneous

