Antiproliferative Illudalane Sesquiterpenes from the Marine Sediment Ascomycete Aspergillus oryzae
- PMID: 34200759
- PMCID: PMC8230370
- DOI: 10.3390/md19060333
Antiproliferative Illudalane Sesquiterpenes from the Marine Sediment Ascomycete Aspergillus oryzae
Abstract
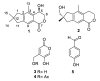
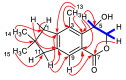
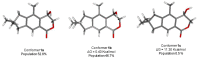
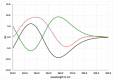
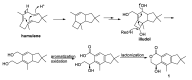
The new asperorlactone (1), along with the known illudalane sesquiterpene echinolactone D (2), two known pyrones, 4-(hydroxymethyl)-5-hydroxy-2H-pyran-2-one (3) and its acetate 4, and 4-hydroxybenzaldehyde (5), were isolated from a culture of Aspergillus oryzae, collected from Red Sea marine sediments. The structure of asperorlactone (1) was elucidated by HR-ESIMS, 1D, and 2D NMR, and a comparison between experimental and DFT calculated electronic circular dichroism (ECD) spectra. This is the first report of illudalane sesquiterpenoids from Aspergillus fungi and, more in general, from ascomycetes. Asperorlactone (1) exhibited antiproliferative activity against human lung, liver, and breast carcinoma cell lines, with IC50 values < 100 µM. All the isolated compounds were also evaluated for their toxicity using the zebrafish embryo model.
Keywords: Aspergillus oryzae; antiproliferative activity; illudalane sesquiterpenes; marine fungus; zebrafish toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Asperienes A-D, Bioactive Sesquiterpenes from the Marine-Derived Fungus Aspergillus flavus.Mar Drugs. 2019 Sep 26;17(10):550. doi: 10.3390/md17100550. Mar Drugs. 2019. PMID: 31561527 Free PMC article.
-
Asporychalasin, a bioactive cytochalasan with an unprecedented 6/6/11 skeleton from the Red Sea sediment Aspergillus oryzae.Phytochemistry. 2021 Dec;192:112952. doi: 10.1016/j.phytochem.2021.112952. Epub 2021 Sep 14. Phytochemistry. 2021. PMID: 34534713
-
Nitrobenzoyl Sesquiterpenoids with Cytotoxic Activities from a Marine-Derived Aspergillus ochraceus Fungus.J Nat Prod. 2018 Jan 26;81(1):92-97. doi: 10.1021/acs.jnatprod.7b00698. Epub 2018 Jan 3. J Nat Prod. 2018. PMID: 29297688
-
Chemical Diversity and Biosynthesis of Drimane-Type Sesquiterpenes in the Fungal Kingdom.Chembiochem. 2022 Sep 5;23(17):e202200173. doi: 10.1002/cbic.202200173. Epub 2022 May 30. Chembiochem. 2022. PMID: 35574818 Free PMC article. Review.
-
Cytotoxic Nitrobenzoyloxy-substituted Sesquiterpenes from Spongederived Endozoic Fungus Aspergillus insulicola MD10-2.Curr Pharm Biotechnol. 2016;17(3):271-4. doi: 10.2174/1389201017666151223123424. Curr Pharm Biotechnol. 2016. PMID: 26696019 Review.
Cited by
-
Research Advances of Bioactive Sesquiterpenoids Isolated from Marine-Derived Aspergillus sp.Molecules. 2022 Oct 30;27(21):7376. doi: 10.3390/molecules27217376. Molecules. 2022. PMID: 36364202 Free PMC article. Review.
-
Two New Lactam Derivatives from Micromelum falcatum (Lour.) Tan. with Brine Shrimp Larvae Toxicity.Molecules. 2023 Oct 18;28(20):7157. doi: 10.3390/molecules28207157. Molecules. 2023. PMID: 37894634 Free PMC article.
-
Aspergillus oryzae as a Cell Factory: Research and Applications in Industrial Production.J Fungi (Basel). 2024 Mar 26;10(4):248. doi: 10.3390/jof10040248. J Fungi (Basel). 2024. PMID: 38667919 Free PMC article. Review.
-
Chemistry and Bioactivity of the Deep-Water Antarctic Octocoral Alcyonium sp.Mar Drugs. 2022 Sep 14;20(9):576. doi: 10.3390/md20090576. Mar Drugs. 2022. PMID: 36135765 Free PMC article.
-
Antimicrobial Activity of Dihydroisocoumarin Isolated from Wadi Lajab Sediment-Derived Fungus Penicillium chrysogenum: In Vitro and In Silico Study.Molecules. 2022 Jun 6;27(11):3630. doi: 10.3390/molecules27113630. Molecules. 2022. PMID: 35684566 Free PMC article.
References
-
- [(accessed on 25 May 2021)]; Available online: https://www.marinepharmacology.org.
-
- White K.M., Rosales R., Yildiz S., Kehrer T., Miorin L., Moreno E., Jangra S., Uccellini M.B., Rathnasinghe R., Coughlan L., et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science. 2021;371:926–931. doi: 10.1126/science.abf4058. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

