Cytomegalovirus Infection and Inflammation in Developing Brain
- PMID: 34200083
- PMCID: PMC8227981
- DOI: 10.3390/v13061078
Cytomegalovirus Infection and Inflammation in Developing Brain
Abstract
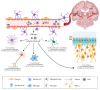
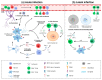
Human cytomegalovirus (HCMV) is a highly prevalent herpesvirus that can cause severe disease in immunocompromised individuals and immunologically immature fetuses and newborns. Most infected newborns are able to resolve the infection without developing sequelae. However, in severe cases, congenital HCMV infection can result in life-threatening pathologies and permanent damage of organ systems that possess a low regenerative capacity. Despite the severity of the problem, HCMV infection of the central nervous system (CNS) remains inadequately characterized to date. Cytomegaloviruses (CMVs) show strict species specificity, limiting the use of HCMV in experimental animals. Infection following intraperitoneal administration of mouse cytomegalovirus (MCMV) into newborn mice efficiently recapitulates many aspects of congenital HCMV infection in CNS. Upon entering the CNS, CMV targets all resident brain cells, consequently leading to the development of widespread histopathology and inflammation. Effector functions from both resident cells and infiltrating immune cells efficiently resolve acute MCMV infection in the CNS. However, host-mediated inflammatory factors can also mediate the development of immunopathologies during CMV infection of the brain. Here, we provide an overview of the cytomegalovirus infection in the brain, local immune response to infection, and mechanisms leading to CNS sequelae.
Keywords: CMV tropism; central nervous system; congenital HCMV infection; cytomegalovirus; immune response; inflammation; latency; mouse cytomegalovirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Susceptibility of Mouse Brain to MCMV Infection and Neuroinflammation During Ontogeny.Pathogens. 2024 Dec 14;13(12):1108. doi: 10.3390/pathogens13121108. Pathogens. 2024. PMID: 39770367 Free PMC article.
-
Human cytomegalovirus glycoprotein variants governing viral tropism and syncytium formation in epithelial cells and macrophages.J Virol. 2024 Jul 23;98(7):e0029324. doi: 10.1128/jvi.00293-24. Epub 2024 Jun 5. J Virol. 2024. PMID: 38837351 Free PMC article.
-
A broadly neutralizing human monoclonal antibody generated from transgenic mice immunized with HCMV particles limits virus infection and proliferation.J Virol. 2024 Jul 23;98(7):e0021324. doi: 10.1128/jvi.00213-24. Epub 2024 Jun 4. J Virol. 2024. PMID: 38832789 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Maribavir treatment for resistant cytomegalovirus disseminated disease in kidney transplant recipients: A case-based scoping review of real life data in literature.Transplant Rev (Orlando). 2024 Dec;38(4):100873. doi: 10.1016/j.trre.2024.100873. Epub 2024 Aug 21. Transplant Rev (Orlando). 2024. PMID: 39178643 Review.
Cited by
-
Neuroinflammation in neurodegeneration via microbial infections.Front Immunol. 2022 Jul 28;13:907804. doi: 10.3389/fimmu.2022.907804. eCollection 2022. Front Immunol. 2022. PMID: 36052093 Free PMC article. Review.
-
SUMOylation and Viral Infections of the Brain.Pathogens. 2022 Jul 21;11(7):818. doi: 10.3390/pathogens11070818. Pathogens. 2022. PMID: 35890062 Free PMC article. Review.
-
The Extraction, Determination, and Bioactivity of Curcumenol: A Comprehensive Review.Molecules. 2024 Jan 30;29(3):656. doi: 10.3390/molecules29030656. Molecules. 2024. PMID: 38338400 Free PMC article. Review.
-
Host-microbe interactions at the blood-brain barrier through the lens of induced pluripotent stem cell-derived brain-like endothelial cells.mBio. 2024 Feb 14;15(2):e0286223. doi: 10.1128/mbio.02862-23. Epub 2024 Jan 9. mBio. 2024. PMID: 38193670 Free PMC article. Review.
-
Prion protein alters viral control and enhances pathology after perinatal cytomegalovirus infection.Nat Commun. 2024 Sep 5;15(1):7754. doi: 10.1038/s41467-024-51931-4. Nat Commun. 2024. PMID: 39237588 Free PMC article.
References
-
- Cannon M.J., Grosse S.D., Fowler K.B. Cytomegaloviruses from Molecular Pathogenesis to Intervention. Caister Academic Press; London, UK: 2013. The Epidemiology and Public Health Impact of Congenital Cytomegalovirus Infec-tion; pp. 26–43.
-
- Boppana S.B., Britt W.J. Cytomegaloviruses from Molec-ular Pathogenesis to Intervention. Caister Academic Press; London, UK: 2013. Synopsis of Clinical Aspects of Human Cytomegalovirus Disease; pp. 1–26.
-
- Schleiss M.R. Current Topics in Microbiology and Immunology. Springer Press; Heidelberg/Berlin, Germany: 2008. Cytomegalovirus Vaccine Development and Target Population Congenital HCMV Infection: A Major Public Health Problem The Problem of Congenital HCMV Infection Is Unquestionably the Major Driving; pp. 361–382. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

