In Vitro and In Silico Anti-Arboviral Activities of Dihalogenated Phenolic Derivates of L-Tyrosine
- PMID: 34198817
- PMCID: PMC8201234
- DOI: 10.3390/molecules26113430
In Vitro and In Silico Anti-Arboviral Activities of Dihalogenated Phenolic Derivates of L-Tyrosine
Abstract
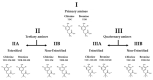
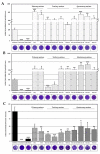
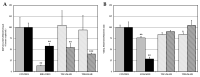
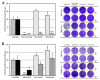
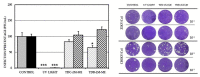
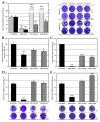
Despite the serious public health problem represented by the diseases caused by dengue (DENV), Zika (ZIKV) and chikungunya (CHIKV) viruses, there are still no specific licensed antivirals available for their treatment. Here, we examined the potential anti-arbovirus activity of ten di-halogenated compounds derived from L-tyrosine with modifications in amine and carboxyl groups. The activity of compounds on VERO cell line infection and the possible mechanism of action of the most promising compounds were evaluated. Finally, molecular docking between the compounds and viral and cellular proteins was evaluated in silico with Autodock Vina®, and the molecular dynamic with Gromacs®. Only two compounds (TDC-2M-ME and TDB-2M-ME) inhibited both ZIKV and CHIKV. Within the possible mechanism, in CHIKV, the two compounds decreased the number of genome copies and in the pre-treatment strategy the infectious viral particles. In the ZIKV model, only TDB-2M-ME inhibited the viral protein and demonstrate a virucidal effect. Moreover, in the U937 cell line infected with CHIKV, both compounds inhibited the viral protein and TDB-2M-ME inhibited the viral genome too. Finally, the in silico results showed a favorable binding energy between the compounds and the helicases of both viral models, the NSP3 of CHIKV and cellular proteins DDC and β2 adrenoreceptor.
Keywords: Zika virus; antiviral agents; chikungunya virus; computational biology; dengue virus; tyrosine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Indole alkaloids inhibit zika and chikungunya virus infection in different cell lines.BMC Complement Med Ther. 2021 Aug 28;21(1):216. doi: 10.1186/s12906-021-03386-z. BMC Complement Med Ther. 2021. PMID: 34454481 Free PMC article.
-
Arctigenin from Arctium lappa L. inhibits chikungunya virus by affecting its entry and replication.Phytomedicine. 2024 Jun;128:155491. doi: 10.1016/j.phymed.2024.155491. Epub 2024 Feb 27. Phytomedicine. 2024. PMID: 38489894
-
Evaluation of the antiviral activity of orlistat (tetrahydrolipstatin) against dengue virus, Japanese encephalitis virus, Zika virus and chikungunya virus.Sci Rep. 2020 Jan 30;10(1):1499. doi: 10.1038/s41598-020-58468-8. Sci Rep. 2020. PMID: 32001767 Free PMC article.
-
Potential Antivirals: Natural Products Targeting Replication Enzymes of Dengue and Chikungunya Viruses.Molecules. 2017 Mar 22;22(3):505. doi: 10.3390/molecules22030505. Molecules. 2017. PMID: 28327521 Free PMC article. Review.
-
Antiviral Role of Phenolic Compounds against Dengue Virus: A Review.Biomolecules. 2020 Dec 24;11(1):11. doi: 10.3390/biom11010011. Biomolecules. 2020. PMID: 33374457 Free PMC article. Review.
Cited by
-
The Mechanism of Action of L-Tyrosine Derivatives against Chikungunya Virus Infection In Vitro Depends on Structural Changes.Int J Mol Sci. 2024 Jul 21;25(14):7972. doi: 10.3390/ijms25147972. Int J Mol Sci. 2024. PMID: 39063216 Free PMC article.
-
Antiviral Profiling of C-18- or C-19-Functionalized Semisynthetic Abietane Diterpenoids.J Nat Prod. 2022 Aug 26;85(8):2044-2051. doi: 10.1021/acs.jnatprod.2c00464. Epub 2022 Aug 15. J Nat Prod. 2022. PMID: 35969814 Free PMC article. Review.
-
Compounds from Natural Products Candidates to Drug for Chikungunya Virus Infection: A Systematic Review.Curr Drug Targets. 2024;25(9):635-648. doi: 10.2174/0113894501304256240524052446. Curr Drug Targets. 2024. PMID: 38847165
-
The Antiviral and Virucidal Activities of Voacangine and Structural Analogs Extracted from Tabernaemontana cymosa Depend on the Dengue Virus Strain.Plants (Basel). 2021 Jun 23;10(7):1280. doi: 10.3390/plants10071280. Plants (Basel). 2021. PMID: 34201900 Free PMC article.
-
Indole alkaloids inhibit zika and chikungunya virus infection in different cell lines.BMC Complement Med Ther. 2021 Aug 28;21(1):216. doi: 10.1186/s12906-021-03386-z. BMC Complement Med Ther. 2021. PMID: 34454481 Free PMC article.
References
-
- Carrillo-Hernández M.Y., Ruiz-Saenz J., Villamizar L.J., Gómez-Rangel S.Y., Martínez-Gutierrez M. Co-circulation and simultaneous co-infection of dengue, chikungunya, and zika viruses in patients with febrile syndrome at the Colombian-Venezuelan border. BMC Infect. Dis. 2018;18:61. doi: 10.1186/s12879-018-2976-1. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

