Ethics of HIV cure research: an unfinished agenda
- PMID: 34193141
- PMCID: PMC8243312
- DOI: 10.1186/s12910-021-00651-1
Ethics of HIV cure research: an unfinished agenda
Abstract
Background: The pursuit of a cure for HIV is a high priority for researchers, funding agencies, governments and people living with HIV (PLWH). To date, over 250 biomedical studies worldwide are or have been related to discovering a safe, effective, and scalable HIV cure, most of which are early translational research and experimental medicine. As HIV cure research increases, it is critical to identify and address the ethical challenges posed by this research.
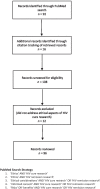
Methods: We conducted a scoping review of the growing HIV cure research ethics literature, focusing on articles published in English peer-reviewed journals from 2013 to 2021. We extracted and summarized key developments in the ethics of HIV cure research. Twelve community advocates actively engaged in HIV cure research provided input on this summary and suggested areas warranting further ethical inquiry and foresight via email exchange and video conferencing.
Discussion: Despite substantial scholarship related to the ethics of HIV cure research, additional attention should focus on emerging issues in six categories of ethical issues: (1) social value (ongoing and emerging biomedical research and scalability considerations); (2) scientific validity (study design issues, such as the use of analytical treatment interruptions and placebos); (3) fair selection of participants (equity and justice considerations); (4) favorable benefit/risk balance (early phase research, benefit-risk balance, risk perception, psychological risks, and pediatric research); (5) informed consent (attention to language, decision-making, informed consent processes and scientific uncertainty); and (6) respect for enrolled participants and community (perspectives of people living with HIV and affected communities and representation).
Conclusion: HIV cure research ethics has an unfinished agenda. Scientific research and bioethics should work in tandem to advance ethical HIV cure research. Because the science of HIV cure research will continue to rapidly advance, ethical considerations of the major themes we identified will need to be revisited and refined over time.
Keywords: Experimental medicine; HIV cure research; People living with HIV; Research ethics.
Conflict of interest statement
Jeremy Sugarman is a member of Merck KGaA’s Bioethics Advisory Panel and Stem Cell Research Oversight Committee; a member of IQVIA’s Ethics Advisory Panel; a member of Aspen Neurosciences Scientific Advisory Board; a member of a Merck Data Monitoring Committee; a consultant to Biogen; and a consultant to Portola Pharmaceuticals Inc. None of these activities are related to the material discussed in this manuscript. No other authors have outside interests to declare.
Figures
Similar articles
-
Ethical and practical considerations for HIV cure-related research at the end-of-life: a qualitative interview and focus group study in the United States.BMC Med Ethics. 2022 Jan 11;23(1):2. doi: 10.1186/s12910-022-00741-8. BMC Med Ethics. 2022. PMID: 35012544 Free PMC article.
-
Synergies, tensions and challenges in HIV prevention, treatment and cure research: exploratory conversations with HIV experts in South Africa.BMC Med Ethics. 2016 Apr 30;17(1):26. doi: 10.1186/s12910-016-0109-1. BMC Med Ethics. 2016. PMID: 27137204 Free PMC article.
-
The psychology of "cure" - unique challenges to consent processes in HIV cure research in South Africa.BMC Med Ethics. 2019 Jan 24;20(1):9. doi: 10.1186/s12910-019-0348-z. BMC Med Ethics. 2019. PMID: 30678664 Free PMC article.
-
Ethical considerations in HIV cure research: points to consider.Curr Opin HIV AIDS. 2013 May;8(3):243-9. doi: 10.1097/COH.0b013e32835ea1c5. Curr Opin HIV AIDS. 2013. PMID: 23422260 Free PMC article. Review.
-
Ethics of HIV and hepatitis B cure research.Curr Opin HIV AIDS. 2020 May;15(3):180-184. doi: 10.1097/COH.0000000000000618. Curr Opin HIV AIDS. 2020. PMID: 32102016 Review.
Cited by
-
A partner protection package for HIV cure-related trials involving analytical treatment interruptions.Lancet Infect Dis. 2023 Oct;23(10):e418-e430. doi: 10.1016/S1473-3099(23)00267-0. Epub 2023 Jun 6. Lancet Infect Dis. 2023. PMID: 37295453 Free PMC article. Review.
-
Centring the health of women across the HIV research continuum.Lancet HIV. 2024 Mar;11(3):e186-e194. doi: 10.1016/S2352-3018(24)00004-3. Lancet HIV. 2024. PMID: 38417977 Free PMC article. Review.
-
Considerations for designing and implementing combination HIV cure trials: findings from a qualitative in-depth interview study in the United States.AIDS Res Ther. 2021 Oct 18;18(1):75. doi: 10.1186/s12981-021-00401-8. AIDS Res Ther. 2021. PMID: 34663375 Free PMC article.
-
A scoping review exploring cure definitions and language for inherited hemoglobinopathies.Genet Med Open. 2024;2:100838. doi: 10.1016/j.gimo.2023.100838. Epub 2023 Oct 27. Genet Med Open. 2024. PMID: 38516178 Free PMC article.
-
Participant experiences in a combination HIV cure-related trial with extended analytical treatment interruption in San Francisco, United States.HIV Res Clin Pract. 2024 Jan 29;25(1):2312318. Epub 2024 Feb 13. HIV Res Clin Pract. 2024. PMID: 38348830 Free PMC article.
References
-
- U.S. DHHS. FDA-Approved HIV Medicines [Internet]. AIDSinfo. 2020 [cited 2020 Apr 28]. Available from: https://aidsinfo.nih.gov/understanding-hiv-aids/fact-sheets/21/58/fda-ap...
-
- TAG. Research Toward a Cure Trials [Internet]. 2021. Available from: http://www.treatmentactiongroup.org/cure/trials.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials