Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine
- PMID: 34192426
- PMCID: PMC8262625
- DOI: 10.1056/NEJMoa2107659
Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine
Abstract
Background: Early clinical data from studies of the NVX-CoV2373 vaccine (Novavax), a recombinant nanoparticle vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that contains the full-length spike glycoprotein of the prototype strain plus Matrix-M adjuvant, showed that the vaccine was safe and associated with a robust immune response in healthy adult participants. Additional data were needed regarding the efficacy, immunogenicity, and safety of this vaccine in a larger population.
Methods: In this phase 3, randomized, observer-blinded, placebo-controlled trial conducted at 33 sites in the United Kingdom, we assigned adults between the ages of 18 and 84 years in a 1:1 ratio to receive two intramuscular 5-μg doses of NVX-CoV2373 or placebo administered 21 days apart. The primary efficacy end point was virologically confirmed mild, moderate, or severe SARS-CoV-2 infection with an onset at least 7 days after the second injection in participants who were serologically negative at baseline.
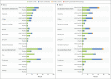
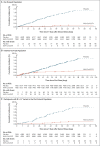
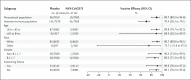
Results: A total of 15,187 participants underwent randomization, and 14,039 were included in the per-protocol efficacy population. Of the participants, 27.9% were 65 years of age or older, and 44.6% had coexisting illnesses. Infections were reported in 10 participants in the vaccine group and in 96 in the placebo group, with a symptom onset of at least 7 days after the second injection, for a vaccine efficacy of 89.7% (95% confidence interval [CI], 80.2 to 94.6). No hospitalizations or deaths were reported among the 10 cases in the vaccine group. Five cases of severe infection were reported, all of which were in the placebo group. A post hoc analysis showed an efficacy of 86.3% (95% CI, 71.3 to 93.5) against the B.1.1.7 (or alpha) variant and 96.4% (95% CI, 73.8 to 99.5) against non-B.1.1.7 variants. Reactogenicity was generally mild and transient. The incidence of serious adverse events was low and similar in the two groups.
Conclusions: A two-dose regimen of the NVX-CoV2373 vaccine administered to adult participants conferred 89.7% protection against SARS-CoV-2 infection and showed high efficacy against the B.1.1.7 variant. (Funded by Novavax; EudraCT number, 2020-004123-16.).
Copyright © 2021 Massachusetts Medical Society.
Figures
Comment in
-
The Novavax vaccine had 90% efficacy against COVID-19 ≥7 d after the second dose.Ann Intern Med. 2021 Nov;174(11):JC124. doi: 10.7326/ACPJ202111160-124. Epub 2021 Nov 2. Ann Intern Med. 2021. PMID: 34724399
Similar articles
-
Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: an exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial.Lancet Respir Med. 2022 Feb;10(2):167-179. doi: 10.1016/S2213-2600(21)00409-4. Epub 2021 Nov 17. Lancet Respir Med. 2022. PMID: 34800364 Free PMC article. Clinical Trial.
-
Safety and immunogenicity following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): a secondary analysis of a randomised, placebo-controlled, phase 2 trial.Lancet Infect Dis. 2022 Nov;22(11):1565-1576. doi: 10.1016/S1473-3099(22)00420-0. Epub 2022 Aug 10. Lancet Infect Dis. 2022. PMID: 35963274 Free PMC article. Clinical Trial.
-
Different dose regimens of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373) in younger and older adults: A phase 2 randomized placebo-controlled trial.PLoS Med. 2021 Oct 1;18(10):e1003769. doi: 10.1371/journal.pmed.1003769. eCollection 2021 Oct. PLoS Med. 2021. PMID: 34597298 Free PMC article. Clinical Trial.
-
Safety, efficacy, and immunogenicity of the NVX-CoV2373 vaccine.Expert Rev Vaccines. 2023 Jan-Dec;22(1):501-517. doi: 10.1080/14760584.2023.2218913. Expert Rev Vaccines. 2023. PMID: 37246757 Review.
-
Comparative efficacy and safety of COVID-19 vaccines in phase III trials: a network meta-analysis.BMC Infect Dis. 2024 Feb 21;24(1):234. doi: 10.1186/s12879-023-08754-3. BMC Infect Dis. 2024. PMID: 38383356 Free PMC article. Review.
Cited by
-
T-Cell Immune Responses to SARS-CoV-2 Infection and Vaccination.Vaccines (Basel). 2024 Sep 30;12(10):1126. doi: 10.3390/vaccines12101126. Vaccines (Basel). 2024. PMID: 39460293 Free PMC article. Review.
-
COVID-19 Vaccination in Kidney Transplant Candidates and Recipients.Vaccines (Basel). 2022 Oct 27;10(11):1808. doi: 10.3390/vaccines10111808. Vaccines (Basel). 2022. PMID: 36366317 Free PMC article.
-
SARS-CoV-2 Infections in Vaccinated and Unvaccinated Populations in Camp Lemonnier, Djibouti, from April 2020 to January 2022.Viruses. 2022 Aug 30;14(9):1918. doi: 10.3390/v14091918. Viruses. 2022. PMID: 36146724 Free PMC article.
-
Viruses, Variants, and Vaccines: How COVID-19 Has Changed the Way We Look at Skin.Curr Dermatol Rep. 2022;11(4):289-312. doi: 10.1007/s13671-022-00370-9. Epub 2022 Oct 17. Curr Dermatol Rep. 2022. PMID: 36274754 Free PMC article. Review.
-
Mapping the intersection of nanotechnology and SARS-CoV-2/COVID-19: A bibliometric analysis.Infect Med (Beijing). 2022 Jun;1(2):103-112. doi: 10.1016/j.imj.2022.06.005. Epub 2022 Jun 26. Infect Med (Beijing). 2022. PMID: 38013718 Free PMC article.
References
-
- World Health Organization. WHO coronavirus disease (COVID-19) dashboard. 2021 (https://covid19.who.int/). - PubMed
-
- Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020;581:215-220. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
