Activated neutrophils polarize protumorigenic interleukin-17A-producing T helper subsets through TNF-α-B7-H2-dependent pathway in human gastric cancer
- PMID: 34185422
- PMCID: PMC8236123
- DOI: 10.1002/ctm2.484
Activated neutrophils polarize protumorigenic interleukin-17A-producing T helper subsets through TNF-α-B7-H2-dependent pathway in human gastric cancer
Abstract
Rationale: Neutrophils constitute massive cellular constituents in inflammatory human gastric cancer (GC) tissues, but their roles in pathogenesis of inflammatory T helper (Th) subsets are still unknown.
Methods: Flow cytometry analysis and immunohistochemistry were used to analyze the responses and phenotypes of neutrophils in different samples from 51 patients with GC. Kaplan-Meier plots and Multivariate analysis for the survival of patients were used by log-rank tests and Cox proportional hazards models. Neutrophils and CD4+ T cells were purified and cultured for ex vivo, in vitro and in vivo regulation and function assays.
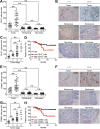
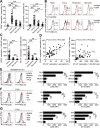
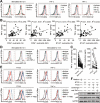
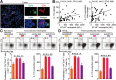
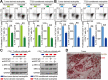
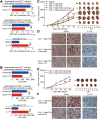
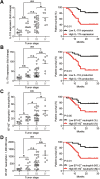
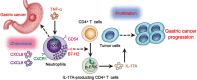
Results: GC patients exhibited increased tumoral neutrophil infiltration with GC progression and poor patient prognosis. Intratumoral neutrophils accumulated in GC tumors via CXCL6/CXCL8-CXCR1-mediated chemotaxis, and expressed activated molecule CD54 and co-signaling molecule B7-H2. Neutrophils induced by tumors strongly expressed CD54 and B7-H2 in both dose- and time-dependent manners, and a close correlation was obtained between the expressions of CD54 and B7-H2 on intratumoral neutrophils. Tumor-derived tumor necrosis factor-α (TNF-α) promoted neutrophil activation and neutrophil B7-H2 expression through ERK-NF-κB pathway, and a significant correlation was found between the levels of TNF-α and CD54+ or B7-H2+ neutrophils in tumor tissues. Tumor-infiltrating and tumor-conditioned neutrophils effectively induced IL-17A-producing Th subset polarization through a B7-H2-dependent manner ex vivo and these polarized IL-17A-producing Th cells exerted protumorigenic roles by promoting GC tumor cell proliferation via inflammatory molecule IL-17A in vitro, which promoted the progression of human GC in vivo; these effects could be reversed when IL-17A is blocked. Moreover, increased B7-H2+ neutrophils and IL-17A in tumors were closely related to advanced GC progression and predicted poor patient survival.
Conclusion: We illuminate novel underlying mechanisms that TNF-α-activated neutrophils link B7-H2 to protumorigenic IL-17A-producing Th subset polarization in human GC. Blocking this pathological TNF-α-B7-H2-IL-17A pathway may be useful therapeutic strategies for treating GC.
Keywords: B7-H2; IL-17A; gastric cancer; neutrophils.
© 2021 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Figures

Similar articles
-
Increased intratumoral mast cells foster immune suppression and gastric cancer progression through TNF-α-PD-L1 pathway.J Immunother Cancer. 2019 Feb 26;7(1):54. doi: 10.1186/s40425-019-0530-3. J Immunother Cancer. 2019. PMID: 30808413 Free PMC article.
-
Granulocyte-Macrophage Colony-Stimulating Factor-Activated Neutrophils Express B7-H4 That Correlates with Gastric Cancer Progression and Poor Patient Survival.J Immunol Res. 2021 Mar 1;2021:6613247. doi: 10.1155/2021/6613247. eCollection 2021. J Immunol Res. 2021. PMID: 33763491 Free PMC article.
-
Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway.Gut. 2017 Nov;66(11):1900-1911. doi: 10.1136/gutjnl-2016-313075. Epub 2017 Mar 8. Gut. 2017. PMID: 28274999 Free PMC article.
-
The roles of IL-17A in inflammatory immune responses and host defense against pathogens.Immunol Rev. 2008 Dec;226:57-79. doi: 10.1111/j.1600-065X.2008.00699.x. Immunol Rev. 2008. PMID: 19161416 Review.
-
Role of interleukin-17 and the neutrophil in asthma.Int Arch Allergy Immunol. 2001 Nov;126(3):179-84. doi: 10.1159/000049511. Int Arch Allergy Immunol. 2001. PMID: 11752873 Review.
Cited by
-
The preoperative scoring system combining neutrophil/lymphocyte ratio and CA19-9 predicts the long-term prognosis of intrahepatic cholangiocarcinoma patients undergoing curative liver resection.BMC Cancer. 2024 Sep 5;24(1):1106. doi: 10.1186/s12885-024-12819-0. BMC Cancer. 2024. PMID: 39237882 Free PMC article.
-
Immunomodulatory Properties of Immune Checkpoint Inhibitors-More than Boosting T-Cell Responses?Cancers (Basel). 2022 Mar 28;14(7):1710. doi: 10.3390/cancers14071710. Cancers (Basel). 2022. PMID: 35406483 Free PMC article. Review.
-
Unveiling the gastric microbiota: implications for gastric carcinogenesis, immune responses, and clinical prospects.J Exp Clin Cancer Res. 2024 Apr 19;43(1):118. doi: 10.1186/s13046-024-03034-7. J Exp Clin Cancer Res. 2024. PMID: 38641815 Free PMC article. Review.
-
Tumor microenvironment-mediated immune tolerance in development and treatment of gastric cancer.Front Immunol. 2022 Oct 20;13:1016817. doi: 10.3389/fimmu.2022.1016817. eCollection 2022. Front Immunol. 2022. PMID: 36341377 Free PMC article. Review.
-
Neutrophils in glioma microenvironment: from immune function to immunotherapy.Front Immunol. 2024 May 8;15:1393173. doi: 10.3389/fimmu.2024.1393173. eCollection 2024. Front Immunol. 2024. PMID: 38779679 Free PMC article. Review.
References
-
- Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric Cancer Lancet. 2016;388:2654‐2664. - PubMed
-
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263‐274. - PubMed
-
- Kuang DM, Peng C, Zhao Q, Wu Y, Chen MS, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology. 2010;51:154‐164. - PubMed
-
- Zhuang Y, Peng LS, Zhao YL, et al. CD8(+) T cells that produce interleukin‐17 regulate myeloid‐derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology. 2012;143:951‐962 e8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
