Study protocol for SKIPMDD: subcutaneous ketamine infusion in palliative care patients with advanced life limiting illnesses for major depressive disorder (phase II pilot feasibility study)
- PMID: 34183351
- PMCID: PMC8240583
- DOI: 10.1136/bmjopen-2021-052312
Study protocol for SKIPMDD: subcutaneous ketamine infusion in palliative care patients with advanced life limiting illnesses for major depressive disorder (phase II pilot feasibility study)
Abstract
Introduction: Major depressive disorder (MDD) in people with advanced life-limiting illnesses can have significant impact on the quality-of-life of those affected. The management of MDD in the palliative care setting can be challenging as typical antidepressants may not work in time nor be tolerated due to coexisting organ dysfunctions, symptom burden and frailty. Parenteral ketamine was found to exhibit effective and rapid-onset antidepressant effect even against treatment-resistant depression in the psychiatric population. However, there is currently neither feasibility study nor available prospective study available to inform of the safety, tolerability and efficacy of such for MDD in the palliative setting.
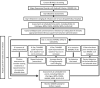
Methods and analysis: This is an open-labelled, single arm, phase II pilot feasibility study involving adult patients with advanced life-limiting illnesses and MDD across four palliative care services in Australia. It has an individual dose-titration design (0.1-0.4 mg/kg) with weekly treatments of subcutaneous ketamine infusion over 2 hours. The primary outcome is feasibility. The secondary outcomes are related to the safety, tolerability and antidepressant efficacy of ketamine, participants' satisfaction in relation to the trial process and the reasons for not completing the study at various stages. The feasibility data will be reported using descriptive statistics. Meanwhile, side effects, tolerability and efficacy data will be analysed using change of assessment scores from baseline.
Ethics and dissemination: Ethics approval was acquired (South Western Sydney Local Health District: HREC/18/LPOOL/466). The results of this study will be submitted for publication in peer-reviewed journals and presented at relevant conferences.
Trial registration number: Australian New Zealand Clinical Trial Registry Number: ACTRN12618001586202; Pre-results.
Keywords: clinical pharmacology; depression & mood disorders; palliative care.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: CL has served on an Advisory Board for Janssen-Cilag and as a consultant for Douglas Pharmaceuticals.
Figures
Similar articles
-
Subcutaneous ketamine infusion in palliative patients for major depressive disorder (SKIPMDD)-Phase II single-arm open-label feasibility study.PLoS One. 2023 Nov 14;18(11):e0290876. doi: 10.1371/journal.pone.0290876. eCollection 2023. PLoS One. 2023. PMID: 37963146 Free PMC article. Clinical Trial.
-
A Phase II, Open-Label Clinical Trial of Intranasal Ketamine for Depression in Patients with Cancer Receiving Palliative Care (INKeD-PC Study).Cancers (Basel). 2023 Jan 7;15(2):400. doi: 10.3390/cancers15020400. Cancers (Basel). 2023. PMID: 36672348 Free PMC article.
-
The Study of Ketamine for Youth Depression (SKY-D): study protocol for a randomised controlled trial of low-dose ketamine for young people with major depressive disorder.Trials. 2023 Oct 24;24(1):686. doi: 10.1186/s13063-023-07631-3. Trials. 2023. PMID: 37875938 Free PMC article.
-
Intravenous ketamine for treatment-resistant major depressive disorder.Ann Pharmacother. 2012 Jan;46(1):117-23. doi: 10.1345/aph.1Q371. Epub 2011 Dec 20. Ann Pharmacother. 2012. PMID: 22190250 Review.
-
Ketamine and other glutamate receptor modulators for depression in adults.Cochrane Database Syst Rev. 2015 Sep 23;(9):CD011612. doi: 10.1002/14651858.CD011612.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2021 Sep 12;9:CD011612. doi: 10.1002/14651858.CD011612.pub3. PMID: 26395901 Updated. Review.
Cited by
-
Subcutaneous ketamine infusion in palliative patients for major depressive disorder (SKIPMDD)-Phase II single-arm open-label feasibility study.PLoS One. 2023 Nov 14;18(11):e0290876. doi: 10.1371/journal.pone.0290876. eCollection 2023. PLoS One. 2023. PMID: 37963146 Free PMC article. Clinical Trial.
-
Caring for depression in the dying is complex and challenging - survey of palliative physicians.BMC Palliat Care. 2022 Jan 16;21(1):11. doi: 10.1186/s12904-022-00901-y. BMC Palliat Care. 2022. PMID: 35034640 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
