Characterisation of a highly potent and near pan-neutralising anti-HIV monoclonal antibody expressed in tobacco plants
- PMID: 34183026
- PMCID: PMC8240387
- DOI: 10.1186/s12977-021-00560-6
Characterisation of a highly potent and near pan-neutralising anti-HIV monoclonal antibody expressed in tobacco plants
Abstract
Background: HIV remains one of the most important health issues worldwide, with almost 40 million people living with HIV. Although patients develop antibodies against the virus, its high mutation rate allows evasion of immune responses. Some patients, however, produce antibodies that are able to bind to, and neutralise different strains of HIV. One such 'broadly neutralising' antibody is 'N6'. Identified in 2016, N6 can neutralise 98% of HIV-1 isolates with a median IC50 of 0.066 µg/mL. This neutralisation breadth makes N6 a very promising therapeutic candidate.
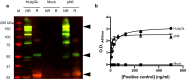
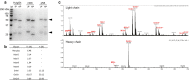
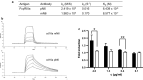
Results: N6 was expressed in a glycoengineered line of N. benthamiana plants (pN6) and compared to the mammalian cell-expressed equivalent (mN6). Expression at 49 mg/kg (fresh leaf tissue) was achieved in plants, although extraction and purification are more challenging than for most plant-expressed antibodies. N-glycoanalysis demonstrated the absence of xylosylation and a reduction in α(1,3)-fucosylation that are typically found in plant glycoproteins. The N6 light chain contains a potential N-glycosylation site, which was modified and displayed more α(1,3)-fucose than the heavy chain. The binding kinetics of pN6 and mN6, measured by surface plasmon resonance, were similar for HIV gp120. pN6 had a tenfold higher affinity for FcγRIIIa, which was reflected in an antibody-dependent cellular cytotoxicity assay, where pN6 induced a more potent response from effector cells than that of mN6. pN6 demonstrated the same potency and breadth of neutralisation as mN6, against a panel of HIV strains.
Conclusions: The successful expression of N6 in tobacco supports the prospect of developing a low-cost, low-tech production platform for a monoclonal antibody cocktail to control HIV in low-to middle income countries.
Keywords: HIV; Immunotherapy; Molecular pharming; Monoclonal antibodies; Plants; bNAbs.
Conflict of interest statement
The authors are aware of no potential conflicts of interest.
Figures


Similar articles
-
HIV-1 bispecific antibody iMab-N6 exhibits enhanced breadth but not potency over its parental antibodies iMab and N6.Virol J. 2022 Sep 7;19(1):143. doi: 10.1186/s12985-022-01876-1. Virol J. 2022. PMID: 36071449 Free PMC article.
-
Plant-based production of highly potent anti-HIV antibodies with engineered posttranslational modifications.Sci Rep. 2020 Apr 10;10(1):6201. doi: 10.1038/s41598-020-63052-1. Sci Rep. 2020. PMID: 32277089 Free PMC article.
-
Enhanced Ability of Plant-Derived PGT121 Glycovariants To Eliminate HIV-1-Infected Cells.J Virol. 2021 Aug 25;95(18):e0079621. doi: 10.1128/JVI.00796-21. Epub 2021 Aug 25. J Virol. 2021. PMID: 34232070 Free PMC article.
-
Brief introduction of current technologies in isolation of broadly neutralizing HIV-1 antibodies.Virus Res. 2018 Jan 2;243:75-82. doi: 10.1016/j.virusres.2017.10.011. Epub 2017 Oct 16. Virus Res. 2018. PMID: 29051051 Free PMC article. Review.
-
To bnAb or Not to bnAb: Defining Broadly Neutralising Antibodies Against HIV-1.Front Immunol. 2021 Oct 19;12:708227. doi: 10.3389/fimmu.2021.708227. eCollection 2021. Front Immunol. 2021. PMID: 34737737 Free PMC article. Review.
Cited by
-
Multiple gene expression in plants using MIDAS-P, a versatile type II restriction-based modular expression vector.Biotechnol Bioeng. 2022 Jun;119(6):1660-1672. doi: 10.1002/bit.28073. Epub 2022 Mar 16. Biotechnol Bioeng. 2022. PMID: 35238400 Free PMC article.
-
Development of plant-made monoclonal antibodies against viral infections.Curr Opin Virol. 2022 Feb;52:148-160. doi: 10.1016/j.coviro.2021.12.005. Epub 2021 Dec 18. Curr Opin Virol. 2022. PMID: 34933212 Free PMC article. Review.
-
HIV-1 bispecific antibody iMab-N6 exhibits enhanced breadth but not potency over its parental antibodies iMab and N6.Virol J. 2022 Sep 7;19(1):143. doi: 10.1186/s12985-022-01876-1. Virol J. 2022. PMID: 36071449 Free PMC article.
-
Harnessing the Potential of Plant Expression System towards the Production of Vaccines for the Prevention of Human Papillomavirus and Cervical Cancer.Vaccines (Basel). 2022 Dec 1;10(12):2064. doi: 10.3390/vaccines10122064. Vaccines (Basel). 2022. PMID: 36560473 Free PMC article. Review.
-
Broadly neutralizing antibodies for HIV prevention: a comprehensive review and future perspectives.Clin Microbiol Rev. 2024 Jun 13;37(2):e0015222. doi: 10.1128/cmr.00152-22. Epub 2024 Apr 30. Clin Microbiol Rev. 2024. PMID: 38687039 Review.
References
-
- WHO | HIV/AIDS. WHO; 2019. https://www.who.int/news-room/fact-sheets/detail/hiv-aids.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

