LncRNA MYLK-AS1 acts as an oncogene by epigenetically silencing large tumor suppressor 2 (LATS2) in gastric cancer
- PMID: 34181498
- PMCID: PMC8806516
- DOI: 10.1080/21655979.2021.1944019
LncRNA MYLK-AS1 acts as an oncogene by epigenetically silencing large tumor suppressor 2 (LATS2) in gastric cancer
Abstract
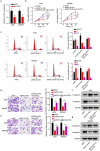
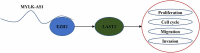
Extensive studies showed the vital function of long noncoding RNAs (lncRNAs) in the pathological and physiological progression of tumors. Previous evidence has indicated that lncRNA MYLK Antisense RNA 1 (MYLK-AS1) acts as an oncogene to facilitate the progression of several tumors. Nevertheless, little is known about its biological role in gastric cancer (GC). Our report intended to probe the underlying mechanism and function of MYLK-AS1 in GC. Results revealed that MYLK-AS1 showed an upregulated level in GC. It was worth mentioning that upregulated MYLK-AS1 caused the unfavorable clinical outcome in GC patients. Functional assays indicated that MYLK-AS1 silencing retarded the proliferation, cell cycle, migration, and invasion in GC. Besides, in vivo assay validated that MYLK-AS1 deficiency also restrained tumor growth. Through in-depth mechanism exploration, MYLK-AS1 was uncovered to bind with wnhancer of zeste homolog 2 (EZH2), an epigenetic inhibitor, to inhibit the level of Large Tumor Suppressor 2 (LATS2), thereby exerting carcinogenicity. Conclusively, our research highlighted the importance of MYLK-AS1 in GC, indicating that MYLK-AS1 might be an effective biomarker for GC.[Figure: see text].
Keywords: EZH2; LATS2; MYLK-AS1; gastric cancer.
Figures

Similar articles
-
Long noncoding RNA DDX11-AS1 epigenetically represses LATS2 by interacting with EZH2 and DNMT1 in hepatocellular carcinoma.Biochem Biophys Res Commun. 2019 Jul 5;514(4):1051-1057. doi: 10.1016/j.bbrc.2019.05.042. Epub 2019 May 13. Biochem Biophys Res Commun. 2019. PMID: 31097223
-
Upregulated long non-coding RNA AGAP2-AS1 represses LATS2 and KLF2 expression through interacting with EZH2 and LSD1 in non-small-cell lung cancer cells.Cell Death Dis. 2016 May 19;7(5):e2225. doi: 10.1038/cddis.2016.126. Cell Death Dis. 2016. PMID: 27195672 Free PMC article.
-
Long noncoding RNA OIP5-AS1 aggravates cell proliferation, migration in gastric cancer by epigenetically silencing NLRP6 expression via binding EZH2.J Cell Biochem. 2020 Jan;121(1):353-362. doi: 10.1002/jcb.29183. Epub 2019 Jun 20. J Cell Biochem. 2020. PMID: 31219209
-
LncRNA SNHG17 promotes gastric cancer progression by epigenetically silencing of p15 and p57.J Cell Physiol. 2019 Apr;234(4):5163-5174. doi: 10.1002/jcp.27320. Epub 2018 Sep 6. J Cell Physiol. 2019. PMID: 30256413
-
Long non-coding RNAs and gastric cancer: An update of potential biomarkers and therapeutic applications.Biomed Pharmacother. 2023 Jul;163:114407. doi: 10.1016/j.biopha.2023.114407. Epub 2023 Apr 24. Biomed Pharmacother. 2023. PMID: 37100014 Review.
Cited by
-
LINC00662 promotes melanoma progression by competitively binding miR-107 and activating the β-catenin signaling pathway.Int J Med Sci. 2024 Jan 1;21(2):265-276. doi: 10.7150/ijms.84072. eCollection 2024. Int J Med Sci. 2024. PMID: 38169586 Free PMC article.
-
LINC01088 regulates the miR-95/LATS2 pathway through the ceRNA mechanism to inhibit the growth, invasion and migration of gastric cancer cells.Int J Immunopathol Pharmacol. 2022 Jan-Dec;36:3946320221108271. doi: 10.1177/03946320221108271. Int J Immunopathol Pharmacol. 2022. PMID: 35728587 Free PMC article.
-
Long non-coding RNA LINC00240 promotes gastric cancer progression via modulating miR-338-5p/METTL3 axis.Bioengineered. 2021 Dec;12(2):9678-9691. doi: 10.1080/21655979.2021.1983276. Bioengineered. 2021. PMID: 34842045 Free PMC article.
-
Targeting the Hippo Pathway in Gastric Cancer and Other Malignancies in the Digestive System: From Bench to Bedside.Biomedicines. 2022 Oct 8;10(10):2512. doi: 10.3390/biomedicines10102512. Biomedicines. 2022. PMID: 36289774 Free PMC article. Review.
-
Identification of Hub lncRNAs Along With lncRNA-miRNA-mRNA Network for Effective Diagnosis and Prognosis of Papillary Thyroid Cancer.Front Pharmacol. 2021 Oct 13;12:748867. doi: 10.3389/fphar.2021.748867. eCollection 2021. Front Pharmacol. 2021. PMID: 34721037 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. . - PubMed
-
- Zong L, Abe M, Seto Y, et al. The challenge of screening for early gastric cancer in China. Lancet. 2016;388(10060):2606. - PubMed
-
- Ponting CP, Oliver PL, Reik W.. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–641. - PubMed
-
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–874. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
