Accuracy of Tau Positron Emission Tomography as a Prognostic Marker in Preclinical and Prodromal Alzheimer Disease: A Head-to-Head Comparison Against Amyloid Positron Emission Tomography and Magnetic Resonance Imaging
- PMID: 34180956
- PMCID: PMC8240013
- DOI: 10.1001/jamaneurol.2021.1858
Accuracy of Tau Positron Emission Tomography as a Prognostic Marker in Preclinical and Prodromal Alzheimer Disease: A Head-to-Head Comparison Against Amyloid Positron Emission Tomography and Magnetic Resonance Imaging
Abstract
Importance: Tau positron emission tomography (PET) tracers have proven useful for the differential diagnosis of dementia, but their utility for predicting cognitive change is unclear.
Objective: To examine the prognostic accuracy of baseline fluorine 18 (18F)-flortaucipir and [18F]RO948 (tau) PET in individuals across the Alzheimer disease (AD) clinical spectrum and to perform a head-to-head comparison against established magnetic resonance imaging (MRI) and amyloid PET markers.
Design, setting, and participants: This prognostic study collected data from 8 cohorts in South Korea, Sweden, and the US from June 1, 2014, to February 28, 2021, with a mean (SD) follow-up of 1.9 (0.8) years. A total of 1431 participants were recruited from memory clinics, clinical trials, or cohort studies; 673 were cognitively unimpaired (CU group; 253 [37.6%] positive for amyloid-β [Aβ]), 443 had mild cognitive impairment (MCI group; 271 [61.2%] positive for Aβ), and 315 had a clinical diagnosis of AD dementia (315 [100%] positive for Aβ).
Exposures: [18F]Flortaucipir PET in the discovery cohort (n = 1135) or [18F]RO948 PET in the replication cohort (n = 296), T1-weighted MRI (n = 1431), and amyloid PET (n = 1329) at baseline and repeated Mini-Mental State Examination (MMSE) evaluation.
Main outcomes and measures: Baseline [18F]flortaucipir/[18F]RO948 PET retention within a temporal region of interest, MRI-based AD-signature cortical thickness, and amyloid PET Centiloids were used to predict changes in MMSE using linear mixed-effects models adjusted for age, sex, education, and cohort. Mediation/interaction analyses tested whether associations between baseline tau PET and cognitive change were mediated by baseline MRI measures and whether age, sex, and APOE genotype modified these associations.
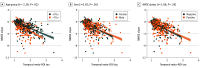
Results: Among 1431 participants, the mean (SD) age was 71.2 (8.8) years; 751 (52.5%) were male. Findings for [18F]flortaucipir PET predicted longitudinal changes in MMSE, and effect sizes were stronger than for AD-signature cortical thickness and amyloid PET across all participants (R2, 0.35 [tau PET] vs 0.24 [MRI] vs 0.17 [amyloid PET]; P < .001, bootstrapped for difference) in the Aβ-positive MCI group (R2, 0.25 [tau PET] vs 0.15 [MRI] vs 0.07 [amyloid PET]; P < .001, bootstrapped for difference) and in the Aβ-positive CU group (R2, 0.16 [tau PET] vs 0.08 [MRI] vs 0.08 [amyloid PET]; P < .001, bootstrapped for difference). These findings were replicated in the [18F]RO948 PET cohort. MRI mediated the association between [18F]flortaucipir PET and MMSE in the groups with AD dementia (33.4% [95% CI, 15.5%-60.0%] of the total effect) and Aβ-positive MCI (13.6% [95% CI, 0.0%-28.0%] of the total effect), but not the Aβ-positive CU group (3.7% [95% CI, -17.5% to 39.0%]; P = .71). Age (t = -2.28; P = .02), but not sex (t = 0.92; P = .36) or APOE genotype (t = 1.06; P = .29) modified the association between baseline [18F]flortaucipir PET and cognitive change, such that older individuals showed faster cognitive decline at similar tau PET levels.
Conclusions and relevance: The findings of this prognostic study suggest that tau PET is a promising tool for predicting cognitive change that is superior to amyloid PET and MRI and may support the prognostic process in preclinical and prodromal stages of AD.
Conflict of interest statement
Figures

Comment in
-
Beyond the AJR: Tau PET, Amyloid PET, and MRI as Prognostic Markers in Early Alzheimer Disease.AJR Am J Roentgenol. 2022 May;218(5):924. doi: 10.2214/AJR.21.26836. Epub 2021 Oct 20. AJR Am J Roentgenol. 2022. PMID: 35234499 No abstract available.
Similar articles
-
Biomarker-Based Prediction of Longitudinal Tau Positron Emission Tomography in Alzheimer Disease.JAMA Neurol. 2022 Feb 1;79(2):149-158. doi: 10.1001/jamaneurol.2021.4654. JAMA Neurol. 2022. PMID: 34928318 Free PMC article.
-
Diagnostic Performance of RO948 F 18 Tau Positron Emission Tomography in the Differentiation of Alzheimer Disease From Other Neurodegenerative Disorders.JAMA Neurol. 2020 Aug 1;77(8):955-965. doi: 10.1001/jamaneurol.2020.0989. JAMA Neurol. 2020. PMID: 32391858 Free PMC article.
-
Discriminative Accuracy of [18F]flortaucipir Positron Emission Tomography for Alzheimer Disease vs Other Neurodegenerative Disorders.JAMA. 2018 Sep 18;320(11):1151-1162. doi: 10.1001/jama.2018.12917. JAMA. 2018. PMID: 30326496 Free PMC article.
-
Comparative Diagnostic Performance of Amyloid-β Positron Emission Tomography and Magnetic Resonance Imaging in Alzheimer's Disease: A Head-to-Head Meta-Analysis.Brain Behav. 2024 Oct;14(10):e70111. doi: 10.1002/brb3.70111. Brain Behav. 2024. PMID: 39435676 Free PMC article.
-
Predicting cognitive decline in older people by structural and molecular imaging.Curr Opin Neurol. 2023 Aug 1;36(4):253-263. doi: 10.1097/WCO.0000000000001172. Epub 2023 Jun 19. Curr Opin Neurol. 2023. PMID: 37382114 Review.
Cited by
-
Discriminative binding of tau PET tracers PI2620, MK6240 and RO948 in Alzheimer's disease, corticobasal degeneration and progressive supranuclear palsy brains.Mol Psychiatry. 2023 Mar;28(3):1272-1283. doi: 10.1038/s41380-022-01875-2. Epub 2022 Nov 29. Mol Psychiatry. 2023. PMID: 36447011 Free PMC article.
-
Exploratory Tau PET/CT with [11C]PBB3 in Patients with Suspected Alzheimer's Disease and Frontotemporal Lobar Degeneration: A Pilot Study on Correlation with PET Imaging and Cerebrospinal Fluid Biomarkers.Biomedicines. 2024 Jul 1;12(7):1460. doi: 10.3390/biomedicines12071460. Biomedicines. 2024. PMID: 39062033 Free PMC article.
-
Comparison of plasma biomarkers and amyloid PET for predicting memory decline in cognitively unimpaired individuals.Alzheimers Dement. 2024 Mar;20(3):2143-2154. doi: 10.1002/alz.13651. Epub 2024 Jan 24. Alzheimers Dement. 2024. PMID: 38265198 Free PMC article.
-
Tau accumulation and its spatial progression across the Alzheimer's disease spectrum.Brain Commun. 2024 Feb 7;6(1):fcae031. doi: 10.1093/braincomms/fcae031. eCollection 2024. Brain Commun. 2024. PMID: 38410618 Free PMC article.
-
Harmonizing tau positron emission tomography in Alzheimer's disease: The CenTauR scale and the joint propagation model.Alzheimers Dement. 2024 Sep;20(9):5833-5848. doi: 10.1002/alz.13908. Epub 2024 Jul 23. Alzheimers Dement. 2024. PMID: 39041435 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

