A Live-Attenuated Equine Influenza Vaccine Stimulates Innate Immunity in Equine Respiratory Epithelial Cell Cultures That Could Provide Protection From Equine Herpesvirus 1
- PMID: 34179166
- PMCID: PMC8224402
- DOI: 10.3389/fvets.2021.674850
A Live-Attenuated Equine Influenza Vaccine Stimulates Innate Immunity in Equine Respiratory Epithelial Cell Cultures That Could Provide Protection From Equine Herpesvirus 1
Abstract
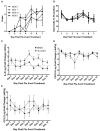
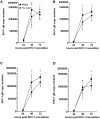
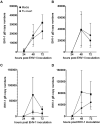
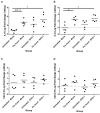
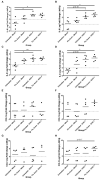
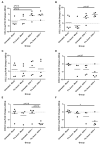
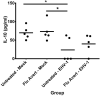
Equine herpesvirus 1 (EHV-1) ubiquitously infects horses worldwide and causes respiratory disease, abortion, and equine herpesvirus myeloencephalopathy. Protection against EHV-1 disease is elusive due to establishment of latency and immune-modulatory features of the virus. These include the modulation of interferons, cytokines, chemokines, antigen presentation, and cellular immunity. Because the modulation of immunity likely occurs at the site of first infection-the respiratory epithelium, we hypothesized that the mucosal influenza vaccine Flu Avert® I.N. (Flu Avert), which is known to stimulate strong antiviral responses, will enhance antiviral innate immunity, and that these responses would also provide protection from EHV-1 infection. To test our hypothesis, primary equine respiratory epithelial cells (ERECs) were treated with Flu Avert, and innate immunity was evaluated for 10 days following treatment. The timing of Flu Avert treatment was also evaluated for optimal effectiveness to reduce EHV-1 replication by modulating early immune responses to EHV-1. The induction of interferons, cytokine and chemokine mRNA expression, and protein secretion was evaluated by high-throughput qPCR and multiplex protein analysis. Intracellular and extracellular EHV-1 titers were determined by qPCR. Flu Avert treatment resulted in the modulation of IL-8, CCL2, and CXCL9 starting at days 5 and 6 post-treatment. Coinciding with the timing of optimal chemokine induction, our data also suggested the same timing for reduction of EHV-1 replication. In combination, our results suggest that Flu Avert may be effective at counteracting some of the immune-modulatory properties of EHV-1 at the airway epithelium and the peak for this response occurs 5-8 days post-Flu Avert treatment. Future in vivo studies are needed to investigate Flu Avert as a prophylactic in situations where EHV-1 exposure may occur.
Keywords: EHV-1; epithelial cell; equine influenza vaccine; horse; mucosal immunity.
Copyright © 2021 Zarski, Vaala, Barnett, Bain and Soboll Hussey.
Conflict of interest statement
WV, DB, and FB are employed by MSD Animal Health. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Innate immune responses of airway epithelial cells to infection with equine herpesvirus-1.Vet Microbiol. 2014 May 14;170(1-2):28-38. doi: 10.1016/j.vetmic.2014.01.018. Epub 2014 Feb 3. Vet Microbiol. 2014. PMID: 24560592
-
An Equine Herpesvirus Type 1 (EHV-1) Ab4 Open Reading Frame 2 Deletion Mutant Provides Immunity and Protection from EHV-1 Infection and Disease.J Virol. 2019 Oct 29;93(22):e01011-19. doi: 10.1128/JVI.01011-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462575 Free PMC article.
-
Equine herpesvirus type 1 pUL56 modulates innate responses of airway epithelial cells.Virology. 2014 Sep;464-465:76-86. doi: 10.1016/j.virol.2014.05.023. Epub 2014 Jul 19. Virology. 2014. PMID: 25046270
-
Equine Herpesvirus-I Infection in Horses: Recent Updates on its Pathogenicity, Vaccination, and Preventive Management Strategies.J Equine Vet Sci. 2020 Apr;87:102923. doi: 10.1016/j.jevs.2020.102923. Epub 2020 Jan 11. J Equine Vet Sci. 2020. PMID: 32172913 Review.
-
The Pathogenesis and Immune Evasive Mechanisms of Equine Herpesvirus Type 1.Front Microbiol. 2021 Mar 4;12:662686. doi: 10.3389/fmicb.2021.662686. eCollection 2021. Front Microbiol. 2021. PMID: 33746936 Free PMC article. Review.
Cited by
-
Associated virus-bacterial vaccine based on seasonal LAIV and S. pneumoniae chimeric peptide provide protection against post-influenza pneumococcal infection in mouse model.Virulence. 2022 Dec;13(1):558-568. doi: 10.1080/21505594.2022.2049496. Virulence. 2022. PMID: 35266442 Free PMC article.
-
Berbamine, a bioactive alkaloid, suppresses equine herpesvirus type 1 in vitro and in vivo.Front Vet Sci. 2023 May 25;10:1163780. doi: 10.3389/fvets.2023.1163780. eCollection 2023. Front Vet Sci. 2023. PMID: 37303732 Free PMC article.
-
Impact of the host immune response on the development of equine herpesvirus myeloencephalopathy in horses.J Gen Virol. 2024 May;105(5):001987. doi: 10.1099/jgv.0.001987. J Gen Virol. 2024. PMID: 38767608 Free PMC article.
References
-
- Gilkerson JR, Whalley JM, Drummer HE, Studdert MJ, Love DN. Epidemiological studies of equine herpesvirus 1 (EHV-1) in thoroughbred foals: a review of studies conducted in the hunter valley of New South Wales between 1995 and 1997. Vet Microbiol. (1999) 68:15–25. 10.1016/S0378-1135(99)00057-7 - DOI - PubMed
-
- Mumford JA, Rossdale PD, Jessett DM, Gann SJ, Ousey J. Serological and virological investigations of an equid herpesvirus 1 (EHV-1) abortion storm on a stud farm in 1985. J Reprod Fertil Suppl. (1987) 35:509–18. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

