Novel therapies targeting hypoxia mechanism to treat pancreatic cancer
- PMID: 34158741
- PMCID: PMC8181875
- DOI: 10.21147/j.issn.1000-9604.2021.02.09
Novel therapies targeting hypoxia mechanism to treat pancreatic cancer
Abstract
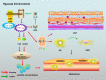
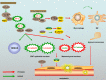
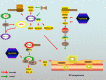
Pancreatic cancer (PC) is one of the deadliest malignancies. The high mortality rate of PC largely results from delayed diagnosis and early metastasis. Therefore, identifying novel treatment targets for patients with PC is urgently required to improve survival rates. A major barrier to successful treatment of PC is the presence of a hypoxic tumor microenvironment, which is associated with poor prognosis, treatment resistance, increased invasion and metastasis. Recent studies have identified a number of novel molecules and pathways in PC cells that promote cancer cells progression under hypoxic conditions, which may provide new therapy strategies to inhibit the development and metastasis of PC. This review summarizes the latest research of hypoxia in PC and provides an overview of how the current therapies have the capacity to overcome hypoxia and improve PC patient treatment. These findings will eventually provide guidance for future PC management and clinical trials and hopefully improve the survival of patients with PC.
Keywords: Pancreatic cancer; hypoxia; novel strategies; survival; tumor microenvironment.
Copyright ©2021Chinese Journal of Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Impact of Hypoxia-Induced miR-210 on Pancreatic Cancer.Curr Issues Mol Biol. 2023 Dec 5;45(12):9778-9792. doi: 10.3390/cimb45120611. Curr Issues Mol Biol. 2023. PMID: 38132457 Free PMC article. Review.
-
MiR-301a transcriptionally activated by HIF-2α promotes hypoxia-induced epithelial-mesenchymal transition by targeting TP63 in pancreatic cancer.World J Gastroenterol. 2020 May 21;26(19):2349-2373. doi: 10.3748/wjg.v26.i19.2349. World J Gastroenterol. 2020. PMID: 32476798 Free PMC article.
-
Pancreatic Cancer Diagnosis and Management: Has the Time Come to Prick the Bubble?Front Endocrinol (Lausanne). 2019 Jan 8;9:779. doi: 10.3389/fendo.2018.00779. eCollection 2018. Front Endocrinol (Lausanne). 2019. PMID: 30671023 Free PMC article. Review.
-
Targeting hypoxic tumor microenvironment in pancreatic cancer.J Hematol Oncol. 2021 Jan 13;14(1):14. doi: 10.1186/s13045-020-01030-w. J Hematol Oncol. 2021. PMID: 33436044 Free PMC article. Review.
-
Hypoxia: a barricade to conquer the pancreatic cancer.Cell Mol Life Sci. 2020 Aug;77(16):3077-3083. doi: 10.1007/s00018-019-03444-3. Epub 2020 Jan 6. Cell Mol Life Sci. 2020. PMID: 31907561 Free PMC article. Review.
Cited by
-
Prognostic Stratification Based on HIF-1 Signaling for Evaluating Hypoxic Status and Immune Infiltration in Pancreatic Ductal Adenocarcinomas.Front Immunol. 2021 Dec 3;12:790661. doi: 10.3389/fimmu.2021.790661. eCollection 2021. Front Immunol. 2021. PMID: 34925373 Free PMC article.
-
Manufacturing CAR-NK against tumors: Who is the ideal supplier?Chin J Cancer Res. 2024 Feb 29;36(1):1-16. doi: 10.21147/j.issn.1000-9604.2024.01.01. Chin J Cancer Res. 2024. PMID: 38455373 Free PMC article.
-
Epidemiology of pancreatic cancer: New version, new vision.Chin J Cancer Res. 2023 Oct 30;35(5):438-450. doi: 10.21147/j.issn.1000-9604.2023.05.03. Chin J Cancer Res. 2023. PMID: 37969957 Free PMC article.
References
LinkOut - more resources
Full Text Sources
