The Atr-Chek1 pathway inhibits axon regeneration in response to Piezo-dependent mechanosensation
- PMID: 34158506
- PMCID: PMC8219705
- DOI: 10.1038/s41467-021-24131-7
The Atr-Chek1 pathway inhibits axon regeneration in response to Piezo-dependent mechanosensation
Abstract
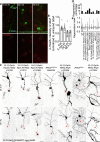
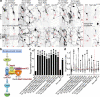
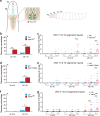
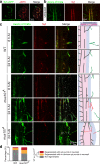
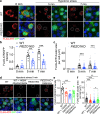
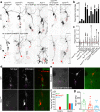
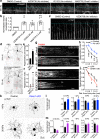
Atr is a serine/threonine kinase, known to sense single-stranded DNA breaks and activate the DNA damage checkpoint by phosphorylating Chek1, which inhibits Cdc25, causing cell cycle arrest. This pathway has not been implicated in neuroregeneration. We show that in Drosophila sensory neurons removing Atr or Chek1, or overexpressing Cdc25 promotes regeneration, whereas Atr or Chek1 overexpression, or Cdc25 knockdown impedes regeneration. Inhibiting the Atr-associated checkpoint complex in neurons promotes regeneration and improves synapse/behavioral recovery after CNS injury. Independent of DNA damage, Atr responds to the mechanical stimulus elicited during regeneration, via the mechanosensitive ion channel Piezo and its downstream NO signaling. Sensory neuron-specific knockout of Atr in adult mice, or pharmacological inhibition of Atr-Chek1 in mammalian neurons in vitro and in flies in vivo enhances regeneration. Our findings reveal the Piezo-Atr-Chek1-Cdc25 axis as an evolutionarily conserved inhibitory mechanism for regeneration, and identify potential therapeutic targets for treating nervous system trauma.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
The Mechanosensitive Ion Channel Piezo Inhibits Axon Regeneration.Neuron. 2019 Apr 17;102(2):373-389.e6. doi: 10.1016/j.neuron.2019.01.050. Epub 2019 Feb 25. Neuron. 2019. PMID: 30819546 Free PMC article.
-
CHEK1 coordinates DNA damage signaling and meiotic progression in the male germline of mice.Hum Mol Genet. 2018 Apr 1;27(7):1136-1149. doi: 10.1093/hmg/ddy022. Hum Mol Genet. 2018. PMID: 29360988 Free PMC article.
-
Duox-generated reactive oxygen species activate ATR/Chk1 to induce G2 arrest in Drosophila tracheoblasts.Elife. 2021 Oct 8;10:e68636. doi: 10.7554/eLife.68636. Elife. 2021. PMID: 34622778 Free PMC article.
-
Interplays between ATM/Tel1 and ATR/Mec1 in sensing and signaling DNA double-strand breaks.DNA Repair (Amst). 2013 Oct;12(10):791-9. doi: 10.1016/j.dnarep.2013.07.009. Epub 2013 Aug 13. DNA Repair (Amst). 2013. PMID: 23953933 Review.
-
Mechanisms of ATR-mediated checkpoint signalling.Front Biosci (Landmark Ed). 2010 Jun 1;15(3):840-53. doi: 10.2741/3649. Front Biosci (Landmark Ed). 2010. PMID: 20515729 Review.
Cited by
-
The mechanism and potential therapeutic target of piezo channels in pain.Front Pain Res (Lausanne). 2024 Sep 27;5:1452389. doi: 10.3389/fpain.2024.1452389. eCollection 2024. Front Pain Res (Lausanne). 2024. PMID: 39398533 Free PMC article. Review.
-
Molecular mechanisms of exercise contributing to tissue regeneration.Signal Transduct Target Ther. 2022 Nov 30;7(1):383. doi: 10.1038/s41392-022-01233-2. Signal Transduct Target Ther. 2022. PMID: 36446784 Free PMC article. Review.
-
Non-canonical role of the ATR pathway in axon regeneration as a mechanosensitive brake.Neural Regen Res. 2022 Nov;17(11):2423-2424. doi: 10.4103/1673-5374.335807. Neural Regen Res. 2022. PMID: 35535883 Free PMC article. No abstract available.
-
Glia instruct axon regeneration via a ternary modulation of neuronal calcium channels in Drosophila.Nat Commun. 2023 Oct 14;14(1):6490. doi: 10.1038/s41467-023-42306-2. Nat Commun. 2023. PMID: 37838791 Free PMC article.
-
A Brief Review on Chemoresistance; Targeting Cancer Stem Cells as an Alternative Approach.Int J Mol Sci. 2023 Feb 24;24(5):4487. doi: 10.3390/ijms24054487. Int J Mol Sci. 2023. PMID: 36901917 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

