PDL1-positive exosomes suppress antitumor immunity by inducing tumor-specific CD8+ T cell exhaustion during metastasis
- PMID: 34152672
- PMCID: PMC8409314
- DOI: 10.1111/cas.15033
PDL1-positive exosomes suppress antitumor immunity by inducing tumor-specific CD8+ T cell exhaustion during metastasis
Abstract
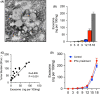
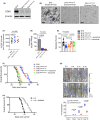
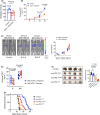
Metastasis is the main cause of death in individuals with cancer. Immune checkpoint blockade (ICB) can potentially reverse CD8+ cytotoxic T lymphocytes (CTLs) dysfunction, leading to significant remission in multiple cancers. However, the mechanism underlying the development of CTL exhaustion during metastatic progression remains unclear. Here, we established an experimental pulmonary metastasis model with melanoma cells and discovered a critical role for melanoma-released exosomes in metastasis. Using genetic knockdown of nSMase2 and Rab27a, 2 key enzymes for exosome secretion, we showed that high levels of effector-like tumor-specific CD8+ T cells with transitory exhaustion, instead of terminal exhaustion, were observed in mice without exosomes; these cells showed limited inhibitory receptors and strong proliferation and cytotoxicity. Mechanistically, the immunosuppression of exosomes depends on exogenous PD-L1, which can be largely rescued by pretreatment with antibody blockade. Notably, we also found that exosomal PD-L1 acts as a promising predictive biomarker for ICB therapies during metastasis. Together, our findings suggest that exosomal PD-L1 may be a potential immunotherapy target, suggesting a new curative therapy for tumor metastasis.
Keywords: exosomes; immune checkpoint blockade therapies; metastasis; predictive biomarker; tumor-specific CD8+ T exhaustion.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
A Small Molecule Antagonist of PD-1/PD-L1 Interactions Acts as an Immune Checkpoint Inhibitor for NSCLC and Melanoma Immunotherapy.Front Immunol. 2021 May 14;12:654463. doi: 10.3389/fimmu.2021.654463. eCollection 2021. Front Immunol. 2021. PMID: 34054817 Free PMC article.
-
Serum-derived exosomal PD-L1 expression to predict anti-PD-1 response and in patients with non-small cell lung cancer.Sci Rep. 2021 Apr 9;11(1):7830. doi: 10.1038/s41598-021-87575-3. Sci Rep. 2021. PMID: 33837261 Free PMC article.
-
Exosomal PD-L1: Roles in Tumor Progression and Immunotherapy.Trends Cancer. 2020 Jul;6(7):550-558. doi: 10.1016/j.trecan.2020.03.002. Epub 2020 Mar 29. Trends Cancer. 2020. PMID: 32610067 Free PMC article. Review.
-
Clinical Implications of Exosomal PD-L1 in Cancer Immunotherapy.J Immunol Res. 2021 Feb 8;2021:8839978. doi: 10.1155/2021/8839978. eCollection 2021. J Immunol Res. 2021. PMID: 33628854 Free PMC article. Review.
-
Transforming growth factor beta orchestrates PD-L1 enrichment in tumor-derived exosomes and mediates CD8 T-cell dysfunction regulating early phosphorylation of TCR signalome in breast cancer.Carcinogenesis. 2021 Feb 11;42(1):38-47. doi: 10.1093/carcin/bgaa092. Carcinogenesis. 2021. PMID: 32832992
Cited by
-
A novel cuproptosis-related prognostic gene signature and validation of differential expression in hepatocellular carcinoma.Front Pharmacol. 2023 Jan 10;13:1081952. doi: 10.3389/fphar.2022.1081952. eCollection 2022. Front Pharmacol. 2023. PMID: 36703728 Free PMC article.
-
Antibody and Cell-Based Therapies against Virus-Induced Cancers in the Context of HIV/AIDS.Pathogens. 2023 Dec 22;13(1):14. doi: 10.3390/pathogens13010014. Pathogens. 2023. PMID: 38251321 Free PMC article. Review.
-
Extracellular vesicle PD-L1 dynamics predict durable response to immune-checkpoint inhibitors and survival in patients with non-small cell lung cancer.J Exp Clin Cancer Res. 2022 Jun 2;41(1):186. doi: 10.1186/s13046-022-02379-1. J Exp Clin Cancer Res. 2022. PMID: 35650597 Free PMC article.
-
Immune cells-derived exosomes function as a double-edged sword: role in disease progression and their therapeutic applications.Biomark Res. 2022 May 12;10(1):30. doi: 10.1186/s40364-022-00374-4. Biomark Res. 2022. PMID: 35550636 Free PMC article. Review.
-
Mechanisms of extracellular vesicle-mediated immune evasion in melanoma.Front Immunol. 2022 Aug 23;13:1002551. doi: 10.3389/fimmu.2022.1002551. eCollection 2022. Front Immunol. 2022. PMID: 36081494 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

