Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Potential Therapeutic Strategy for Acute Kidney Injury
- PMID: 34149726
- PMCID: PMC8209464
- DOI: 10.3389/fimmu.2021.684496
Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Potential Therapeutic Strategy for Acute Kidney Injury
Abstract
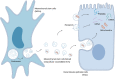
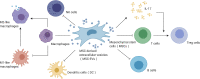
Acute kidney injury (AKI) is a common and potential life-threatening disease in patients admitted to hospital, affecting 10%-15% of all hospitalizations and around 50% of patients in the intensive care unit. Severe, recurrent, and uncontrolled AKI may progress to chronic kidney disease or end-stage renal disease. AKI thus requires more efficient, specific therapies, rather than just supportive therapy. Mesenchymal stem cells (MSCs) are considered to be promising cells for cellular therapy because of their ease of harvesting, low immunogenicity, and ability to expand in vitro. Recent research indicated that the main therapeutic effects of MSCs were mediated by MSC-derived extracellular vesicles (MSC-EVs). Furthermore, compared with MSCs, MSC-EVs have lower immunogenicity, easier storage, no tumorigenesis, and the potential to be artificially modified. We reviewed the therapeutic mechanism of MSCs and MSC-EVs in AKI, and considered recent research on how to improve the efficacy of MSC-EVs in AKI. We also summarized and analyzed the potential and limitations of EVs for the treatment of AKI to provide ideas for future clinical trials and the clinical application of MSC-EVs in AKI.
Keywords: acute kidney injury; cytokine; extracellular vesicle; mesenchymal stem cell; tubular epithelial cell.
Copyright © 2021 Li, Yang, Su, Luo, Luo, Huang, Tu and Luo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor has declared past co-authorships with one of the authors (CY) in the time of review.
Figures
Similar articles
-
Molecular Mechanisms of Mesenchymal Stem Cell-Based Therapy in Acute Kidney Injury.Int J Mol Sci. 2021 Oct 22;22(21):11406. doi: 10.3390/ijms222111406. Int J Mol Sci. 2021. PMID: 34768837 Free PMC article. Review.
-
The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury.Stem Cell Res Ther. 2017 Feb 7;8(1):24. doi: 10.1186/s13287-017-0478-5. Stem Cell Res Ther. 2017. PMID: 28173878 Free PMC article.
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles to the Rescue of Renal Injury.Int J Mol Sci. 2021 Jun 20;22(12):6596. doi: 10.3390/ijms22126596. Int J Mol Sci. 2021. PMID: 34202940 Free PMC article. Review.
-
Oct-4 Enhanced the Therapeutic Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Acute Kidney Injury.Kidney Blood Press Res. 2020;45(1):95-108. doi: 10.1159/000504368. Epub 2020 Jan 10. Kidney Blood Press Res. 2020. PMID: 31927554
-
Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges.Stem Cell Res Ther. 2017 Dec 4;8(1):273. doi: 10.1186/s13287-017-0727-7. Stem Cell Res Ther. 2017. PMID: 29202871 Free PMC article. Review.
Cited by
-
Dual Role of Extracellular Vesicles in Sepsis-Associated Kidney and Lung Injury.Biomedicines. 2022 Sep 30;10(10):2448. doi: 10.3390/biomedicines10102448. Biomedicines. 2022. PMID: 36289710 Free PMC article. Review.
-
Mesenchymal Stromal Cell-Derived Extracellular Vesicles as Biological Carriers for Drug Delivery in Cancer Therapy.Front Bioeng Biotechnol. 2022 Apr 14;10:882545. doi: 10.3389/fbioe.2022.882545. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35497332 Free PMC article. Review.
-
Mesenchymal stem cell transplantation alleviated atherosclerosis in systemic lupus erythematosus through reducing MDSCs.Stem Cell Res Ther. 2022 Jul 18;13(1):328. doi: 10.1186/s13287-022-03002-y. Stem Cell Res Ther. 2022. PMID: 35850768 Free PMC article.
-
Cellular nanovesicles for therapeutic immunomodulation: A perspective on engineering strategies and new advances.Acta Pharm Sin B. 2023 May;13(5):1789-1827. doi: 10.1016/j.apsb.2022.08.020. Epub 2022 Aug 28. Acta Pharm Sin B. 2023. PMID: 37250173 Free PMC article. Review.
-
Fatty Acid β-Oxidation in Kidney Diseases: Perspectives on Pathophysiological Mechanisms and Therapeutic Opportunities.Front Pharmacol. 2022 Apr 20;13:805281. doi: 10.3389/fphar.2022.805281. eCollection 2022. Front Pharmacol. 2022. PMID: 35517820 Free PMC article. Review.
References
-
- Pickkers P, Mehta RL, Murray PT, Joannidis M, Molitoris BA, Kellum JA, et al. . Effect of Human Recombinant Alkaline Phosphatase on 7-Day Creatinine Clearance in Patients With Sepsis-Associated Acute Kidney Injury: A Randomized Clinical Trial. Jama (2018) 320:1998–2009. 10.1001/jama.2018.14283 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources