Prospective Clinical, Virologic, and Immunologic Assessment of COVID-19 in Transplant Recipients
- PMID: 34149003
- PMCID: PMC8487707
- DOI: 10.1097/TP.0000000000003860
Prospective Clinical, Virologic, and Immunologic Assessment of COVID-19 in Transplant Recipients
Abstract
Background: Several studies have described the clinical features of COVID-19 in solid-organ transplant recipients. However, many have been retrospective or limited to more severe cases (hospitalized) and have not routinely included serial virological sampling (especially in outpatients) and immunologic assessment.
Methods: Transplant patients diagnosed with COVID-19 based on a respiratory sample PCR were prospectively followed up to 90 d. Patients provided consent for convalescent serum samples and serial nasopharyngeal swabs for SARS-CoV-2 antibody (antinucleoprotein and anti-RBD) and viral load, respectively.
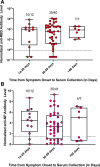
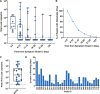
Results: In the 161 SOT recipients diagnosed with COVID-19, the spectrum of disease ranged from asymptomatic infection (4.3%) to hospitalization (60.6%), supplemental oxygen requirement (43.1%), mechanical ventilation (22.7%), and death (15.6%). Increasing age (OR, 1.031; 95% CI, 1.001-1.062; P = 0.046) and ≥2 comorbid conditions (OR, 3.690; 95% CI, 1.418-9.615; P = 0.007) were associated with the need for supplemental oxygen. Allograft rejection was uncommon (3.7%) despite immunosuppression modification. Antibody response at ≥14 d postsymptoms onset was present in 90% (anti-RBD) and 76.7% (anti-NP) with waning of anti-NP titers and stability of anti-RBD over time. Median duration of nasopharyngeal positivity was 10.0 d (IQR, 5.5-18.0) and shedding beyond 30 d was observed in 6.7% of patients. The development of antibody did not have an impact on viral shedding.
Conclusions: This study demonstrates the spectrum of COVID-19 illness in transplant patients. Risk factors for severe disease are identified. The majority form antibody by 2 wk with differential stability over time. Prolonged viral shedding was observed in a minority of patients. Reduction of immunosuppression was a safe strategy.
Copyright © 2021 Wolters Kluwer Health, Inc. All rights reserved.
Figures
Similar articles
-
Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study.Lancet Infect Dis. 2020 May;20(5):565-574. doi: 10.1016/S1473-3099(20)30196-1. Epub 2020 Mar 23. Lancet Infect Dis. 2020. PMID: 32213337 Free PMC article.
-
Viral and Antibody Kinetics of COVID-19 Patients with Different Disease Severities in Acute and Convalescent Phases: A 6-Month Follow-Up Study.Virol Sin. 2020 Dec;35(6):820-829. doi: 10.1007/s12250-020-00329-9. Epub 2020 Dec 22. Virol Sin. 2020. PMID: 33351168 Free PMC article.
-
In-depth virological assessment of kidney transplant recipients with COVID-19.Am J Transplant. 2020 Nov;20(11):3162-3172. doi: 10.1111/ajt.16251. Epub 2020 Sep 12. Am J Transplant. 2020. PMID: 32777130 Free PMC article.
-
Clinical implications of SARS-CoV-2 cycle threshold values in solid organ transplant recipients.Am J Transplant. 2021 Mar;21(3):1304-1311. doi: 10.1111/ajt.16357. Epub 2020 Oct 31. Am J Transplant. 2021. PMID: 33043603 Free PMC article.
-
The Immunology of SARS-CoV-2 Infection and Vaccines in Solid Organ Transplant Recipients.Viruses. 2021 Sep 20;13(9):1879. doi: 10.3390/v13091879. Viruses. 2021. PMID: 34578460 Free PMC article. Review.
Cited by
-
Severity of COVID-19 among solid organ transplant recipients in Canada, 2020-2021: a prospective, multicentre cohort study.CMAJ. 2022 Aug 29;194(33):E1155-E1163. doi: 10.1503/cmaj.220620. CMAJ. 2022. PMID: 36302101 Free PMC article.
-
Severe Acute Respiratory Syndrome Coronavirus 2 Infection Induces Greater T-Cell Responses Compared to Vaccination in Solid Organ Transplant Recipients.J Infect Dis. 2021 Dec 1;224(11):1849-1860. doi: 10.1093/infdis/jiab542. J Infect Dis. 2021. PMID: 34739078 Free PMC article.
-
Homotypic and heterotypic immune responses to Omicron variant in immunocompromised patients in diverse clinical settings.Nat Commun. 2022 Aug 4;13(1):4489. doi: 10.1038/s41467-022-32235-x. Nat Commun. 2022. PMID: 35927279 Free PMC article.
-
Viral cultures, cycle threshold values and viral load estimation for assessing SARS-CoV-2 infectiousness in haematopoietic stem cell and solid organ transplant patients: a systematic review.J Hosp Infect. 2023 Feb;132:62-72. doi: 10.1016/j.jhin.2022.11.018. Epub 2022 Dec 5. J Hosp Infect. 2023. PMID: 36473552 Free PMC article.
-
Outcomes of SARS-CoV-2 Infection in Unvaccinated Compared With Vaccinated Solid Organ Transplant Recipients: A Propensity Matched Cohort Study.Transplantation. 2022 Aug 1;106(8):1622-1628. doi: 10.1097/TP.0000000000004178. Epub 2022 May 3. Transplantation. 2022. PMID: 35502801 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

