Therapeutic uses of post-partum tissue-derived mesenchymal stromal cell secretome
- PMID: 34145093
- PMCID: PMC8224162
- DOI: 10.4103/ijmr.IJMR_1450_18
Therapeutic uses of post-partum tissue-derived mesenchymal stromal cell secretome
Abstract
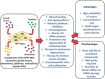
Human post-partum tissue mesenchymal stromal cells (hPPT-MSCs) are widely used in research to investigate their differentiation capabilities and therapeutic effects as potential agents in cell-based therapy. This is ascribed to the advantages offered by the use of MSCs isolated from hPPT over other MSC sources. A paradigm shift in related research is evident that focuses on the secretome of the human MSCs (hMSCs), as therapeutic effects of hMSCs are attributed more so to their secreted growth factors, cytokines and chemokines and to the extracellular vesicles (EVs), all of which are components of the hMSC secretome. Positive therapeutic effects of the hPPT-MSC secretome have been demonstrated in diseases related to skin, kidney, heart, nervous system, cartilage and bones, that have aided fast recovery by replacing damaged, non-functional tissues, via differentiating and regenerating cells. Although certain limitations such as short half -life of the secretome components and irregular secreting patterns exist in secretome therapy, these issues are successfully addressed with the use of cutting-edge technologies such as genome editing and recombinant cytokine treatment. If the current limitations can be successfully overcome, the hPPT-MSC secretome including its EVs may be developed into a cost-effective therapeutic agent amenable to be used against a wide range of diseases/disorders.
Keywords: Extracellular vesicles; human mesenchymal stromal cell secretome; post-partum tissue; stem cell therapy.
Conflict of interest statement
None
Figures
Similar articles
-
An overview of the current advances in the treatment of inflammatory diseases using mesenchymal stromal cell secretome.Immunopharmacol Immunotoxicol. 2023 Dec;45(4):497-507. doi: 10.1080/08923973.2023.2180388. Epub 2023 Feb 28. Immunopharmacol Immunotoxicol. 2023. PMID: 36786742 Review.
-
Secretome Derived from Mesenchymal Stem/Stromal Cells: A Promising Strategy for Diabetes and its Complications.Curr Stem Cell Res Ther. 2024;19(10):1328-1350. doi: 10.2174/1574888X19666230913154544. Curr Stem Cell Res Ther. 2024. PMID: 37711134 Review.
-
Effects of Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Lung Diseases: Current Status and Future Perspectives.Stem Cell Rev Rep. 2021 Apr;17(2):440-458. doi: 10.1007/s12015-020-10085-8. Epub 2020 Nov 19. Stem Cell Rev Rep. 2021. PMID: 33211245 Free PMC article. Review.
-
Inflammatory priming enhances mesenchymal stromal cell secretome potential as a clinical product for regenerative medicine approaches through secreted factors and EV-miRNAs: the example of joint disease.Stem Cell Res Ther. 2020 Apr 28;11(1):165. doi: 10.1186/s13287-020-01677-9. Stem Cell Res Ther. 2020. PMID: 32345351 Free PMC article.
-
Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis.Cell Tissue Res. 2018 Oct;374(1):1-15. doi: 10.1007/s00441-018-2871-5. Epub 2018 Jun 28. Cell Tissue Res. 2018. PMID: 29955951 Review.
Cited by
-
The role of primed and non-primed MSC-derived conditioned media in neuroregeneration.Front Mol Neurosci. 2023 Nov 2;16:1241432. doi: 10.3389/fnmol.2023.1241432. eCollection 2023. Front Mol Neurosci. 2023. PMID: 38025267 Free PMC article.
References
-
- Strauer BE, Kornowski R. Stem cell therapy in perspective. Circulation. 2003;107:929–34. - PubMed
-
- Rajput BS, Chakrabarti SK, Dongare VS, Ramirez CM, Deb KD. Human umbilical cord mesenchymal stem cells in the treatment of duchenne muscular dystrophy: Safety and feasibility study in India. J Stem Cells. 2015;10:141–56. - PubMed
-
- Squillaro T, Peluso G, Galderisi U. Clinical Trials with mesenchymal stem cells: An Update. Cell Transplant. 2016;25:829–48. - PubMed
-
- Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 2008;100:133–58. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

