Developmental Differences in Neocortex Neurogenesis and Maturation Between the Altricial Dwarf Rabbit and Precocial Guinea Pig
- PMID: 34135738
- PMCID: PMC8200626
- DOI: 10.3389/fnana.2021.678385
Developmental Differences in Neocortex Neurogenesis and Maturation Between the Altricial Dwarf Rabbit and Precocial Guinea Pig
Abstract
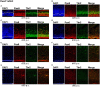
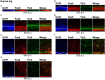
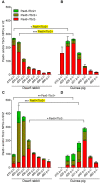
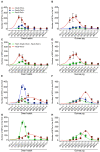
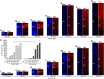
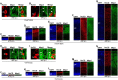
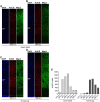
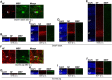
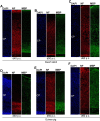
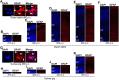
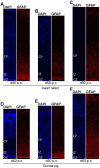
Mammals are born on a precocial-altricial continuum. Altricial species produce helpless neonates with closed distant organs incapable of locomotion, whereas precocial species give birth to well-developed young that possess sophisticated sensory and locomotor capabilities. Previous studies suggest that distinct patterns of cortex development differ between precocial and altricial species. This study compares patterns of neocortex neurogenesis and maturation in the precocial guinea pig and altricial dwarf rabbit, both belonging to the taxon of Glires. We show that the principal order of neurodevelopmental events is preserved in the neocortex of both species. Moreover, we show that neurogenesis starts at a later postconceptional day and takes longer in absolute gestational days in the precocial than the altricial neocortex. Intriguingly, our data indicate that the dwarf rabbit neocortex contains a higher abundance of highly proliferative basal progenitors than the guinea pig, which might underlie its higher encephalization quotient, demonstrating that the amount of neuron production is determined by complex regulation of multiple factors. Furthermore, we show that the guinea pig neocortex exhibits a higher maturation status at birth, thus providing evidence for the notions that precocial species might have acquired the morphological machinery required to attain their high functional state at birth and that brain expansion in the precocial newborn is mainly due to prenatally initiating processes of gliogenesis and neuron differentiation instead of increased neurogenesis. Together, this study reveals important insights into the timing and cellular differences that regulate mammalian brain growth and maturation and provides a better understanding of the evolution of mammalian altriciality and presociality.
Keywords: altricial; cortex development; dwarf rabbit; guinea pig; neurogenesis; neuron maturation; precocial.
Copyright © 2021 Kalusa, Heinrich, Sauerland, Morawski and Fietz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Neocortex neurogenesis and maturation in the African greater cane rat.Neural Dev. 2023 Oct 13;18(1):7. doi: 10.1186/s13064-023-00175-x. Neural Dev. 2023. PMID: 37833718 Free PMC article.
-
Timing and Distribution of Mitotic Activity in the Retina During Precocial and Altricial Modes of Avian Development.Front Neurosci. 2022 May 9;16:853544. doi: 10.3389/fnins.2022.853544. eCollection 2022. Front Neurosci. 2022. PMID: 35615284 Free PMC article.
-
Comparative anatomy of neonates of the three major mammalian groups (monotremes, marsupials, placentals) and implications for the ancestral mammalian neonate morphotype.J Anat. 2017 Dec;231(6):798-822. doi: 10.1111/joa.12689. Epub 2017 Sep 28. J Anat. 2017. PMID: 28960296 Free PMC article.
-
Early ontogeny of locomotor behaviour: a comparison between altricial and precocial animals.Brain Res Bull. 2000 Nov 15;53(5):719-26. doi: 10.1016/s0361-9230(00)00404-4. Brain Res Bull. 2000. PMID: 11165806 Review.
-
Metabolism and energetics of avian embryos.J Exp Zool Suppl. 1987;1:111-25. J Exp Zool Suppl. 1987. PMID: 3298529 Review.
Cited by
-
Reduced neural progenitor cell count and cortical neurogenesis in guinea pigs congenitally infected with Toxoplasma gondii.Commun Biol. 2023 Nov 27;6(1):1209. doi: 10.1038/s42003-023-05576-6. Commun Biol. 2023. PMID: 38012384 Free PMC article.
-
Dual isolation of primary neurons and oligodendrocytes from guinea pig frontal cortex.Front Cell Neurosci. 2024 Jan 10;17:1298685. doi: 10.3389/fncel.2023.1298685. eCollection 2023. Front Cell Neurosci. 2024. PMID: 38269115 Free PMC article.
-
It is time to explore the impact of length of gestation and fetal health on the human lifespan.Aging Cell. 2024 Apr;23(4):e14157. doi: 10.1111/acel.14157. Epub 2024 Apr 1. Aging Cell. 2024. PMID: 38558485 Free PMC article.
-
Placental vascular alterations are associated with early neurodevelopmental and pulmonary impairment in the rabbit fetal growth restriction model.Sci Rep. 2022 Nov 16;12(1):19720. doi: 10.1038/s41598-022-22895-6. Sci Rep. 2022. PMID: 36385147 Free PMC article.
-
Neocortex neurogenesis and maturation in the African greater cane rat.Neural Dev. 2023 Oct 13;18(1):7. doi: 10.1186/s13064-023-00175-x. Neural Dev. 2023. PMID: 37833718 Free PMC article.
References
-
- Asher M., De Oliveira E. S., Sachser N. (2004). Social system and spatial organization of wild guinea pigs (Cavia aperea) in a natural population. J. Mammal. 85, 788–796. 10.1644/BNS-012 - DOI
-
- Attardo A., Calegari F., Haubensak W., Wilsch-Brauninger M., Huttner W. B. (2008). Live imaging at the onset of cortical neurogenesis reveals differential appearance of the neuronal phenotype in apical versus basal progenitor progeny. PLoS ONE 3:e2388. 10.1371/journal.pone.0002388 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

