Murine Cytomegalovirus MCK-2 Facilitates In Vivo Infection Transfer from Dendritic Cells to Salivary Gland Acinar Cells
- PMID: 34132572
- PMCID: PMC8354226
- DOI: 10.1128/JVI.00693-21
Murine Cytomegalovirus MCK-2 Facilitates In Vivo Infection Transfer from Dendritic Cells to Salivary Gland Acinar Cells
Abstract
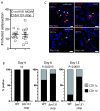
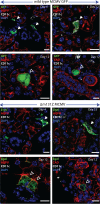
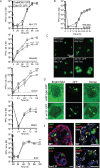
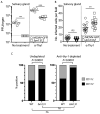
The cytomegaloviruses (CMVs) spread systemically via myeloid cells and demonstrate broad tissue tropism. Human CMV (HCMV) UL128 encodes a component of the virion pentameric complex (PC) that is important for entry into epithelial cells and cell-cell spread in vitro. It possesses N-terminal amino acid sequences similar to those of CC chemokines. While the species specificity of HCMV precludes confirmation of UL128 function in vivo, UL128-like counterparts in experimental animals have demonstrated a role in salivary gland infection. How they achieve this has not been defined, although effects on monocyte tropism and immune evasion have been proposed. By tracking infected cells following lung infection, we show that although the UL128-like protein in mouse CMV (MCMV) (designated MCK-2) facilitated entry into lung macrophages, it was dispensable for subsequent viremia mediated by CD11c+ dendritic cells (DCs) and extravasation to the salivary glands. Notably, MCK-2 was important for the transfer of MCMV infection from DCs to salivary gland acinar epithelial cells. Acinar cell infection of MCMVs deleted of MCK-2 was not rescued by T-cell depletion, arguing against an immune evasion mechanism for MCK-2 in the salivary glands. In contrast to lung infection, peritoneal MCMV inoculation yields mixed monocyte/DC viremia. In this setting, MCK-2 again promoted DC-dependent infection of salivary gland acinar cells, but it was not required for monocyte-dependent spread to the lung. Thus, the action of MCK-2 in MCMV spread was specific to DC-acinar cell interactions. IMPORTANCE Cytomegaloviruses (CMVs) establish myeloid cell-associated viremias and persistent shedding from the salivary glands. In vitro studies with human CMV (HCMV) have implicated HCMV UL128 in epithelial tropism, but its role in vivo is unknown. Here, we analyzed how a murine CMV (MCMV) protein with similar physical properties, designated MCK-2, contributes to host colonization. We demonstrate that MCK-2 is dispensable for initial systemic spread from primary infection sites but within the salivary gland facilitates the transfer of infection from dendritic cells (DCs) to epithelial acinar cells. Virus transfer from extravasated monocytes to the lungs did not require MCK-2, indicating a tissue-specific effect. These results provide new information about how persistent viral tropism determinants operate in vivo.
Keywords: chemokines; dendritic cells; mouse cytomegalovirus.
Figures

Similar articles
-
Mouse cytomegalovirus lacking sgg1 shows reduced import into the salivary glands.J Gen Virol. 2024 Aug;105(8):002013. doi: 10.1099/jgv.0.002013. J Gen Virol. 2024. PMID: 39093048
-
Murine Cytomegalovirus Spreads by Dendritic Cell Recirculation.mBio. 2017 Oct 3;8(5):e01264-17. doi: 10.1128/mBio.01264-17. mBio. 2017. PMID: 28974616 Free PMC article.
-
The viral chemokine MCK-2 of murine cytomegalovirus promotes infection as part of a gH/gL/MCK-2 complex.PLoS Pathog. 2013;9(7):e1003493. doi: 10.1371/journal.ppat.1003493. Epub 2013 Jul 25. PLoS Pathog. 2013. PMID: 23935483 Free PMC article.
-
Dendritic cells in cytomegalovirus infection: viral evasion and host countermeasures.APMIS. 2009 May;117(5-6):413-26. doi: 10.1111/j.1600-0463.2009.02449.x. APMIS. 2009. PMID: 19400865 Review.
-
Cytomegalovirus host entry and spread.J Gen Virol. 2019 Apr;100(4):545-553. doi: 10.1099/jgv.0.001230. Epub 2019 Feb 7. J Gen Virol. 2019. PMID: 30730289 Review.
Cited by
-
CD4+ T Cells Control Murine Cytomegalovirus Infection Indirectly.J Virol. 2022 Apr 13;96(7):e0007722. doi: 10.1128/jvi.00077-22. Epub 2022 Mar 16. J Virol. 2022. PMID: 35293772 Free PMC article.
-
Multiple Autonomous Cell Death Suppression Strategies Ensure Cytomegalovirus Fitness.Viruses. 2021 Aug 27;13(9):1707. doi: 10.3390/v13091707. Viruses. 2021. PMID: 34578288 Free PMC article.
-
Recent Advancements in Understanding Primary Cytomegalovirus Infection in a Mouse Model.Viruses. 2022 Aug 31;14(9):1934. doi: 10.3390/v14091934. Viruses. 2022. PMID: 36146741 Free PMC article. Review.
-
Dissecting the cytomegalovirus CC chemokine: Chemokine activity and gHgLchemokine-dependent cell tropism are independent players in CMV infection.PLoS Pathog. 2023 Dec 8;19(12):e1011793. doi: 10.1371/journal.ppat.1011793. eCollection 2023 Dec. PLoS Pathog. 2023. PMID: 38064525 Free PMC article.
-
The Mouse Cytomegalovirus G Protein-Coupled Receptor Homolog, M33, Coordinates Key Features of In Vivo Infection via Distinct Components of Its Signaling Repertoire.J Virol. 2022 Feb 23;96(4):e0186721. doi: 10.1128/JVI.01867-21. Epub 2021 Dec 8. J Virol. 2022. PMID: 34878888 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

