The Classical, Yet Controversial, First Enzyme of Lipid Synthesis: Escherichia coli Acetyl-CoA Carboxylase
- PMID: 34132100
- PMCID: PMC8483704
- DOI: 10.1128/MMBR.00032-21
The Classical, Yet Controversial, First Enzyme of Lipid Synthesis: Escherichia coli Acetyl-CoA Carboxylase
Abstract
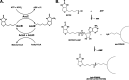
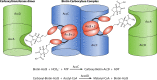
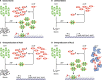
Escherichia coli acetyl-CoA carboxylase (ACC), the enzyme responsible for synthesis of malonyl-CoA, the building block of fatty acid synthesis, is the paradigm bacterial ACC. Many reports on the structures and stoichiometry of the four subunits comprising the active enzyme as well as on regulation of ACC activity and expression have appeared in the almost 20 years since this subject was last reviewed. This review seeks to update and expand on these reports.
Keywords: acetyl-CoA; biotin; carboxylase; fatty acid synthesis; malonyl-CoA.
Figures
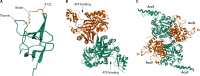
Similar articles
-
Acetyl-CoA carboxylase from Escherichia coli exhibits a pronounced hysteresis when inhibited by palmitoyl-acyl carrier protein.Arch Biochem Biophys. 2017 Dec 15;636:100-109. doi: 10.1016/j.abb.2017.10.016. Epub 2017 Oct 31. Arch Biochem Biophys. 2017. PMID: 29100983
-
Complex formation and regulation of Escherichia coli acetyl-CoA carboxylase.Biochemistry. 2013 May 14;52(19):3346-57. doi: 10.1021/bi4000707. Epub 2013 May 1. Biochemistry. 2013. PMID: 23594205
-
The bacterial signal transduction protein GlnB regulates the committed step in fatty acid biosynthesis by acting as a dissociable regulatory subunit of acetyl-CoA carboxylase.Mol Microbiol. 2015 Mar;95(6):1025-35. doi: 10.1111/mmi.12912. Epub 2015 Jan 30. Mol Microbiol. 2015. PMID: 25557370
-
Multi-subunit acetyl-CoA carboxylases.Prog Lipid Res. 2002 Sep;41(5):407-35. doi: 10.1016/s0163-7827(02)00007-3. Prog Lipid Res. 2002. PMID: 12121720 Review.
-
Engineering intracellular malonyl-CoA availability in microbial hosts and its impact on polyketide and fatty acid synthesis.Appl Microbiol Biotechnol. 2020 Jul;104(14):6057-6065. doi: 10.1007/s00253-020-10643-7. Epub 2020 May 8. Appl Microbiol Biotechnol. 2020. PMID: 32385515 Free PMC article. Review.
Cited by
-
Medium-Chain-Length Fatty Acid Catabolism in Cupriavidus necator H16: Transcriptome Sequencing Reveals Differences from Long-Chain-Length Fatty Acid β-Oxidation and Involvement of Several Homologous Genes.Appl Environ Microbiol. 2023 Jan 31;89(1):e0142822. doi: 10.1128/aem.01428-22. Epub 2022 Dec 21. Appl Environ Microbiol. 2023. PMID: 36541797 Free PMC article.
-
Structural assembly of the bacterial essential interactome.Elife. 2024 Jan 16;13:e94919. doi: 10.7554/eLife.94919. Elife. 2024. PMID: 38226900 Free PMC article.
-
Enhanced biosynthesis of poly(3-hydroxybutyrate) in engineered strains of Pseudomonas putida via increased malonyl-CoA availability.Microb Biotechnol. 2024 Nov;17(11):e70044. doi: 10.1111/1751-7915.70044. Microb Biotechnol. 2024. PMID: 39503721 Free PMC article.
-
Clostridioides difficile exploits xanthine and uric acid as nutrients by utilizing a selenium-dependent catabolic pathway.Microbiol Spectr. 2024 Oct 3;12(10):e0084424. doi: 10.1128/spectrum.00844-24. Epub 2024 Aug 21. Microbiol Spectr. 2024. PMID: 39166854 Free PMC article.
-
An inhibitory mechanism of AasS, an exogenous fatty acid scavenger: Implications for re-sensitization of FAS II antimicrobials.PLoS Pathog. 2024 Jul 15;20(7):e1012376. doi: 10.1371/journal.ppat.1012376. eCollection 2024 Jul. PLoS Pathog. 2024. PMID: 39008531 Free PMC article.
References
-
- Guchhait RB, Polakis SE, Dimroth P, Stoll E, Moss J, Lane MD. 1974. Acetyl coenzyme A carboxylase system of Escherichia coli. Purification and properties of the biotin carboxylase, carboxyltransferase, and carboxyl carrier protein components. J Biol Chem 249:6633–6645. 10.1016/S0021-9258(19)42203-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

