Child outcomes after induction of labour or expectant management in women with preterm prelabour rupture of membranes between 34 and 37 weeks of gestation: study protocol of the PPROMEXIL Follow-up trial. A long-term follow-up study of the randomised controlled trials PPROMEXIL and PPROMEXIL-2
- PMID: 34130959
- PMCID: PMC8208011
- DOI: 10.1136/bmjopen-2020-046046
Child outcomes after induction of labour or expectant management in women with preterm prelabour rupture of membranes between 34 and 37 weeks of gestation: study protocol of the PPROMEXIL Follow-up trial. A long-term follow-up study of the randomised controlled trials PPROMEXIL and PPROMEXIL-2
Abstract
Introduction: Late preterm prelabour rupture of membranes (PROM between 34+0 and 36+6 weeks gestational age) is an important clinical dilemma. Previously, two large Dutch randomised controlled trials (RCTs) compared induction of labour (IoL) to expectant management (EM). Both trials showed that early delivery does not reduce the risk of neonatal sepsis as compared with EM, although prematurity-related risks might increase. An extensive, structured long-term follow-up of these children has never been performed.
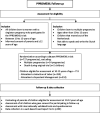
Methods and analysis: The PPROMEXIL Follow-up trial (NL6623 (NTR6953)) aims to assess long-term childhood outcomes of the PPROMEXIL (ISRCTN29313500) and PPROMEXIL-2 trial (ISRCTN05689407), two multicentre RCTs using the same protocol, conducted between 2007 and 2010 evaluating IoL versus EM in women with late preterm PROM. The PPROMEXIL Follow-up will analyse children of mothers with a singleton pregnancy (PPROMEXIL trial n=520, PPROMEXIL-2 trial n=191, total IoL n=359; total EM n=352). At 10-12 years of age all surviving children will be invited for a neurodevelopmental assessment using the Wechsler Intelligence Scale for Children-V, Color-Word Interference Test and the Movement Assessment Battery for Children-2. Parents will be asked to fill out questionnaires assessing behaviour, motor function, sensory processing, respiratory problems, general health and need for healthcare services. Teachers will fill out the Teacher Report Form and answer questions regarding school attainment. For all tests means with SDs will be compared, as well as predefined cut-off scores for abnormal outcome. Sensitivity analyses consisting of different imputation techniques will be used to deal with lost to follow-up.
Ethics and dissemination: The study has been granted approval by the Medical Centre Amsterdam (MEC) of the AmsterdamUMC (MEC2016_217). Results will be disseminated through peer-reviewed journals and summaries shared with stakeholders. This protocol is published before analysis of the results.
Trial registration number: NL6623 (NTR6953).
Keywords: developmental neurology & neurodisability; fetal medicine; maternal medicine; prenatal diagnosis.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: BWM is supported by an NHMRC Practitioner Fellowship (GNT1082548). BWM reports consultancy for ObsEva, Merck Merck KGaA and Guerbet. All other authors did not report any conflicts of interest.
Figures
Similar articles
-
Childhood outcomes after induction of labor or expectant management for preterm prelabor rupture of membranes: a 10-year follow-up of the PPROMEXIL trials.Am J Obstet Gynecol. 2023 May;228(5):588.e1-588.e13. doi: 10.1016/j.ajog.2023.02.007. Epub 2023 Feb 12. Am J Obstet Gynecol. 2023. PMID: 36787813
-
Induction of labour versus expectant management in women with preterm prelabour rupture of membranes between 34 and 37 weeks (the PPROMEXIL-trial).BMC Pregnancy Childbirth. 2007 Jul 6;7:11. doi: 10.1186/1471-2393-7-11. BMC Pregnancy Childbirth. 2007. PMID: 17617892 Free PMC article. Clinical Trial.
-
Behavioural and neurodevelopmental outcome of 2-year-old children after preterm premature rupture of membranes: follow-up of a randomised clinical trial comparing induction of labour and expectant management.Eur J Obstet Gynecol Reprod Biol. 2015 Nov;194:17-23. doi: 10.1016/j.ejogrb.2015.07.014. Epub 2015 Aug 3. Eur J Obstet Gynecol Reprod Biol. 2015. PMID: 26319651 Clinical Trial.
-
Preterm and term prelabour rupture of membranes: A review of timing and methods of labour induction.Best Pract Res Clin Obstet Gynaecol. 2021 Nov;77:27-41. doi: 10.1016/j.bpobgyn.2021.08.009. Epub 2021 Sep 3. Best Pract Res Clin Obstet Gynaecol. 2021. PMID: 34538740 Review.
-
Planned early birth versus expectant management (waiting) for prelabour rupture of membranes at term (37 weeks or more).Cochrane Database Syst Rev. 2006 Jan 25;(1):CD005302. doi: 10.1002/14651858.CD005302.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2017 Jan 04;1:CD005302. doi: 10.1002/14651858.CD005302.pub3 PMID: 16437525 Updated. Review.
Cited by
-
Pessary or progesterone to prevent preterm birth in women with short cervical length: protocol of the 4-6 year follow-up of a randomised controlled trial (Quadruple-P).BMJ Open. 2022 Aug 24;12(8):e064049. doi: 10.1136/bmjopen-2022-064049. BMJ Open. 2022. PMID: 36002221 Free PMC article.
-
Atosiban versus placebo in the treatment of threatened preterm birth between 30 and 34 weeks gestation: study protocol of the 4-year APOSTEL 8 follow-up.BMJ Open. 2024 Jul 18;14(7):e083600. doi: 10.1136/bmjopen-2023-083600. BMJ Open. 2024. PMID: 39025819 Free PMC article.
-
Diagnostic accuracy of ultrasound screening for fetal structural abnormalities during the first and second trimester of pregnancy in low-risk and unselected populations.Cochrane Database Syst Rev. 2024 May 9;5(5):CD014715. doi: 10.1002/14651858.CD014715.pub2. Cochrane Database Syst Rev. 2024. PMID: 38721874 Review.
References
-
- Nederlandse Vereniging voor Obstetrie en Gynaecologie [Rupture of membranes before onset of labor. Available: http://nvog-documenten.nl/
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources