Systematic review and meta analysis of differential attrition between active and control arms in randomized controlled trials of lifestyle interventions in chronic disease
- PMID: 34126934
- PMCID: PMC8204467
- DOI: 10.1186/s12874-021-01313-x
Systematic review and meta analysis of differential attrition between active and control arms in randomized controlled trials of lifestyle interventions in chronic disease
Abstract
Background: Attrition is a major obstacle for lifestyle interventions sustained for the medium-to-long term and can have significant consequences on the internal validity of a trial. When the degree of attrition differs between active and control arms this is termed differential attrition and is an important consideration during initial stages of trial planning.
Objectives: The primary research question of this study was: what is the differential attrition between treatment arms in lifestyle interventions for prevalent chronic diseases?
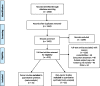
Methods: We performed a systematic review and meta-analysis of 23 studies involving a lifestyle intervention component in cohorts with chronic diseases. The search accessed three databases: Scopus, Medline Ovid and Web of Science. Attrition between treatment arms was analysed using a random-effects model and examined the relationship between the relative attrition and potential moderators, such as time to final follow-up, time to first follow-up, type of disease, type of control, type of intervention and length of treatment.
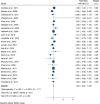
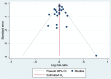
Results: The pooled risk ratio was 1.00 (95% CI 0.97 - 1.03) and only one study fell outside this range. A univariable association was described between the pooled risk ration and length (years) to final follow-up, which did not remain in the multivariable model.
Conclusions: Ultimately, we found no evidence of differential attrition in medium-to-long term lifestyle intervention studies for chronic disease, increasing confidence in conducting such studies with minimal potential of attrition bias.
Trial registration: PROSPERO registration number CRD42018084495 .
Keywords: Attrition; Chronic disease; Lifestyle; Retention.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: An Updated Systematic Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. PMID: 30354042 Free Books & Documents. Review.
-
Differential attrition in health behaviour change trials: a systematic review and meta-analysis.Psychol Health. 2015 Jan;30(1):122-34. doi: 10.1080/08870446.2014.953526. Psychol Health. 2015. PMID: 25109224 Review.
-
Lifestyle intervention for improving school achievement in overweight or obese children and adolescents.Cochrane Database Syst Rev. 2014 Mar 14;(3):CD009728. doi: 10.1002/14651858.CD009728.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2018 Jan 29;1:CD009728. doi: 10.1002/14651858.CD009728.pub3. PMID: 24627300 Updated. Review.
-
The effect of weight management interventions that include a diet component on weight-related outcomes in pregnant and postpartum women: a systematic review protocol.JBI Database System Rev Implement Rep. 2015 Jan;13(1):88-98. doi: 10.11124/jbisrir-2015-1812. JBI Database System Rev Implement Rep. 2015. PMID: 26447010
-
Systematic review and meta-analysis of the impact of preconception lifestyle interventions on fertility, obstetric, fetal, anthropometric and metabolic outcomes in men and women.Hum Reprod. 2017 Sep 1;32(9):1925-1940. doi: 10.1093/humrep/dex241. Hum Reprod. 2017. PMID: 28854715 Review.
Cited by
-
Study protocol for an online lifestyle modification education course for people living with multiple sclerosis: the multiple sclerosis online course (MSOC).BMC Neurol. 2023 Jun 29;23(1):249. doi: 10.1186/s12883-023-03298-0. BMC Neurol. 2023. PMID: 37386385 Free PMC article.
References
-
- Rabinowitz J, Levine SZ, Barkai O, Davidov O. Dropout rates in randomized clinical trials of antipsychotics: a meta-analysis comparing first- and second-generation drugs and an examination of the role of trial design features. Schizophr Bull. 2009;35(4):775–788. doi: 10.1093/schbul/sbn005. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources