G2/M Checkpoint Abrogation With Selective Inhibitors Results in Increased Chromatid Breaks and Radiosensitization of 82-6 hTERT and RPE Human Cells
- PMID: 34123995
- PMCID: PMC8193504
- DOI: 10.3389/fpubh.2021.675095
G2/M Checkpoint Abrogation With Selective Inhibitors Results in Increased Chromatid Breaks and Radiosensitization of 82-6 hTERT and RPE Human Cells
Abstract
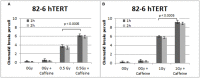
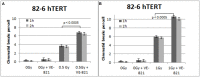
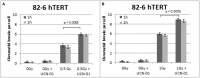
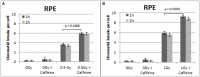
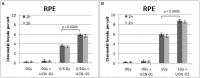
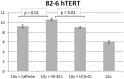
While technological advances in radiation oncology have led to a more precise delivery of radiation dose and a decreased risk of side effects, there is still a need to better understand the mechanisms underlying DNA damage response (DDR) at the DNA and cytogenetic levels, and to overcome tumor resistance. To maintain genomic stability, cells have developed sophisticated signaling pathways enabling cell cycle arrest to facilitate DNA repair via the DDR-related kinases and their downstream targets, so that DNA damage or DNA replication stress induced by genotoxic therapies can be resolved. ATM, ATR, and Chk1 kinases are key mediators in DDR activation and crucial factors in treatment resistance. It is of importance, therefore, as an alternative to the conventional clonogenic assay, to establish a cytogenetic assay enabling reliable and time-efficient results in evaluating the potency of DDR inhibitors for radiosensitization. Toward this goal, the present study aims at the development and optimization of a chromosomal radiosensitivity assay using the DDR and G2-checkpoint inhibitors as a novel modification compared to the classical G2-assay. Also, it aims at investigating the strengths of this assay for rapid radiosensitivity assessments in cultured cells, and potentially, in tumor cells obtained from biopsies. Specifically, exponentially growing RPE and 82-6 hTERT human cells are irradiated during the G2/M-phase transition in the presence or absence of Caffeine, VE-821, and UCN-1 inhibitors of ATM/ATR, ATR, and Chk1, respectively, and the induced chromatid breaks are used to evaluate cell radiosensitivity and their potency for radiosensitization. The increased yield of chromatid breaks in the presence of DDR inhibitors, which underpins radiosensitization, is similar to that observed in cells from highly radiosensitive AT-patients, and is considered here as 100% radiosensitive internal control. The results highlight the potential of our modified G2-assay using VE-821 to evaluate cell radiosensitivity, the efficacy of DDR inhibitors in radiosensitization, and reinforce the concept that ATM, ATR, and Chk1 represent attractive anticancer drug targets in radiation oncology.
Keywords: DDR inhibitors; G2-M checkpoint; G2-assay; chromatid breaks; chromosomal radiosensitivity.
Copyright © 2021 Nikolakopoulou, Soni, Habibi, Karaiskos, Pantelias, Terzoudi and Iliakis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Depletion of ATR selectively sensitizes ATM-deficient human mammary epithelial cells to ionizing radiation and DNA-damaging agents.Cell Cycle. 2014;13(22):3541-50. doi: 10.4161/15384101.2014.960729. Cell Cycle. 2014. PMID: 25483091 Free PMC article.
-
G2-checkpoint abrogation in irradiated lymphocytes: A new cytogenetic approach to assess individual radiosensitivity and predisposition to cancer.Int J Oncol. 2009 Nov;35(5):1223-30. doi: 10.3892/ijo_00000439. Int J Oncol. 2009. PMID: 19787278
-
A standardized G2-assay for the prediction of individual radiosensitivity.Radiother Oncol. 2011 Oct;101(1):28-34. doi: 10.1016/j.radonc.2011.09.021. Epub 2011 Oct 18. Radiother Oncol. 2011. PMID: 22014898
-
ATM and ATR as therapeutic targets in cancer.Pharmacol Ther. 2015 May;149:124-38. doi: 10.1016/j.pharmthera.2014.12.001. Epub 2014 Dec 13. Pharmacol Ther. 2015. PMID: 25512053 Review.
-
Potentiation of DNA-damage-induced cytotoxicity by G2 checkpoint abrogators.Curr Med Chem Anticancer Agents. 2003 Jan;3(1):35-46. doi: 10.2174/1568011033353533. Curr Med Chem Anticancer Agents. 2003. PMID: 12678913 Review.
Cited by
-
A specific dispiropiperazine derivative that arrests cell cycle, induces apoptosis, necrosis and DNA damage.Sci Rep. 2023 May 29;13(1):8674. doi: 10.1038/s41598-023-35927-6. Sci Rep. 2023. PMID: 37248333 Free PMC article.
-
Replication Stress: A Review of Novel Targets to Enhance Radiosensitivity-From Bench to Clinic.Front Oncol. 2022 Jul 8;12:838637. doi: 10.3389/fonc.2022.838637. eCollection 2022. Front Oncol. 2022. PMID: 35875060 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

