Combination of chemotherapy and oxidative stress to enhance cancer cell apoptosis
- PMID: 34122827
- PMCID: PMC8157308
- DOI: 10.1039/c9sc05997k
Combination of chemotherapy and oxidative stress to enhance cancer cell apoptosis
Abstract
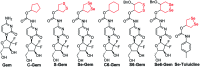
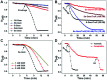
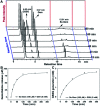
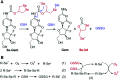
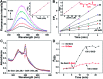
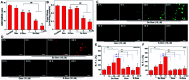
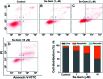
Cancer cells are vulnerable to reactive oxygen species (ROS) due to their abnormal redox environment. Accordingly, combination of chemotherapy and oxidative stress has gained increasing interest for the treatment of cancer. We report a novel seleno-prodrug of gemcitabine (Gem), Se-Gem, and evaluated its activation and biological effects in cancer cells. Se-Gem was prepared by introducing a 1,2-diselenolane (a five-membered cyclic diselenide) moiety into the parent drug Gem via a carbamate linker. Se-Gem is preferably activated by glutathione (GSH) and displays a remarkably higher potency than Gem (up to a 6-fold increase) to a panel of cancer cell lines. The activation of Se-Gem by GSH releases Gem and a seleno-intermediate nearly quantitatively. Unlike the most ignored side products in prodrug activation, the seleno-intermediate further catalyzes a conversion of GSH and oxygen to GSSG (oxidized GSH) and ROS via redox cycling reactions. Thus Se-Gem may be considered as a suicide agent to deplete GSH and works by a combination of chemotherapy and oxidative stress. This is the first case that employs a cyclic diselenide in prodrug design, and the success of Se-Gem as well as its well-defined action mechanism demonstrates that the 1,2-diselenolane moiety may serve as a general scaffold to advance constructing novel therapeutic molecules with improved potency via a combination of chemotherapy and oxidative stress.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
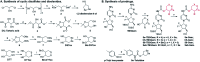
Similar articles
-
Role of selenium toxicity and oxidative stress in aquatic birds.Aquat Toxicol. 2002 Apr;57(1-2):11-26. doi: 10.1016/s0166-445x(01)00263-6. Aquat Toxicol. 2002. PMID: 11879935 Review.
-
Dual role of glutathione in selenite-induced oxidative stress and apoptosis in human hepatoma cells.Free Radic Biol Med. 2000 Apr 1;28(7):1115-24. doi: 10.1016/s0891-5849(00)00206-9. Free Radic Biol Med. 2000. PMID: 10832073
-
Glutathione--linking cell proliferation to oxidative stress.Free Radic Biol Med. 2015 Dec;89:1154-64. doi: 10.1016/j.freeradbiomed.2015.09.023. Epub 2015 Nov 3. Free Radic Biol Med. 2015. PMID: 26546102 Review.
-
ROS/KRAS/AMPK Signaling Contributes to Gemcitabine-Induced Stem-like Cell Properties in Pancreatic Cancer.Mol Ther Oncolytics. 2019 Aug 9;14:299-312. doi: 10.1016/j.omto.2019.07.005. eCollection 2019 Sep 27. Mol Ther Oncolytics. 2019. PMID: 31508487 Free PMC article.
-
A preclinical evaluation of pemetrexed and irinotecan combination as second-line chemotherapy in pancreatic cancer.Br J Cancer. 2007 May 7;96(9):1358-67. doi: 10.1038/sj.bjc.6603726. Epub 2007 Apr 10. Br J Cancer. 2007. PMID: 17426706 Free PMC article.
Cited by
-
Onopordopicrin from the new genus Shangwua as a novel thioredoxin reductase inhibitor to induce oxidative stress-mediated tumor cell apoptosis.J Enzyme Inhib Med Chem. 2021 Dec;36(1):790-801. doi: 10.1080/14756366.2021.1899169. J Enzyme Inhib Med Chem. 2021. PMID: 33733960 Free PMC article.
-
Combination of metformin/efavirenz/fluoxetine exhibits profound anticancer activity via a cancer cell-specific ROS amplification.Cancer Biol Ther. 2023 Dec 31;24(1):20-32. doi: 10.1080/15384047.2022.2161803. Cancer Biol Ther. 2023. PMID: 36588385 Free PMC article.
-
Multifaceted role of redox pattern in the tumor immune microenvironment regarding autophagy and apoptosis.Mol Cancer. 2023 Aug 10;22(1):130. doi: 10.1186/s12943-023-01831-w. Mol Cancer. 2023. PMID: 37563639 Free PMC article. Review.
-
The Cellular Origins of Cancer-Associated Fibroblasts and Their Opposing Contributions to Pancreatic Cancer Growth.Front Cell Dev Biol. 2021 Sep 27;9:743907. doi: 10.3389/fcell.2021.743907. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34646829 Free PMC article. Review.
-
Fluorescent Probes for Mammalian Thioredoxin Reductase: Mechanistic Analysis, Construction Strategies, and Future Perspectives.Biosensors (Basel). 2023 Aug 13;13(8):811. doi: 10.3390/bios13080811. Biosensors (Basel). 2023. PMID: 37622897 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

