Association of Premature Immune Aging and Cytomegalovirus After Solid Organ Transplant
- PMID: 34122420
- PMCID: PMC8190404
- DOI: 10.3389/fimmu.2021.661551
Association of Premature Immune Aging and Cytomegalovirus After Solid Organ Transplant
Abstract
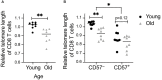
Immune function is altered with increasing age. Infection with cytomegalovirus (CMV) accelerates age-related immunological changes resulting in expanded oligoclonal memory CD8 T cell populations with impaired proliferation, signaling, and cytokine production. As a consequence, elderly CMV seropositive (CMV+) individuals have increased mortality and impaired responses to other infections in comparison to seronegative (CMV-) individuals of the same age. CMV is also a significant complication after organ transplantation, and recent studies have shown that CMV-associated expansion of memory T cells is accelerated after transplantation. Thus, we investigated whether immune aging is accelerated post-transplant, using a combination of telomere length, flow cytometry phenotyping, and single cell RNA sequencing. Telomere length decreased slightly in the first year after transplantation in a subset of both CMV+ and CMV- recipients with a strong concordance between CD57+ cells and short telomeres. Phenotypically aged cells increased post-transplant specifically in CMV+ recipients, and clonally expanded T cells were enriched for terminally differentiated cells post-transplant. Overall, these findings demonstrate a pattern of accelerated aging of the CD8 T cell compartment in CMV+ transplant recipients.
Keywords: Telomere; cytomegalovirus (CMV); flow cytometry; immunosenescence; transplantation immunobiology.
Copyright © 2021 Higdon, Gustafson, Ji, Sahoo, Pinsky, Margulies, Maecker, Goronzy and Maltzman.
Conflict of interest statement
JSM has a family member who is employed by and has an equity interest in Genentech/Roche. No patents have been filed pertaining to the results presented in this paper. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Multifunctional cytomegalovirus (CMV)-specific CD8(+) T cells are not restricted by telomere-related senescence in young or old adults.Immunology. 2015 Apr;144(4):549-60. doi: 10.1111/imm.12409. Immunology. 2015. PMID: 25314332 Free PMC article.
-
Cytomegalovirus contributes partly to uraemia-associated premature immunological ageing of the T cell compartment.Clin Exp Immunol. 2013 Dec;174(3):424-32. doi: 10.1111/cei.12188. Clin Exp Immunol. 2013. PMID: 23962178 Free PMC article.
-
CMV drives the expansion of highly functional memory T cells expressing NK-cell receptors in renal transplant recipients.Eur J Immunol. 2017 Aug;47(8):1324-1334. doi: 10.1002/eji.201747018. Epub 2017 Jul 3. Eur J Immunol. 2017. PMID: 28586095
-
Impact of cytomegalovirus on early immunosenescence of CD8+ T lymphocytes after solid organ transplantation.J Gerontol A Biol Sci Med Sci. 2013 Jan;68(1):1-5. doi: 10.1093/gerona/gls130. Epub 2012 May 2. J Gerontol A Biol Sci Med Sci. 2013. PMID: 22552369 Review.
-
Analyzing T-cell responses to cytomegalovirus by cytokine flow cytometry.Hum Immunol. 2004 May;65(5):493-9. doi: 10.1016/j.humimm.2004.02.004. Hum Immunol. 2004. PMID: 15172449 Review.
Cited by
-
CXCL8, CCL2, and CMV Seropositivity as New Prognostic Factors for a Severe COVID-19 Course.Int J Mol Sci. 2022 Sep 26;23(19):11338. doi: 10.3390/ijms231911338. Int J Mol Sci. 2022. PMID: 36232655 Free PMC article.
-
The Entangled World of Memory T Cells and Implications in Transplantation.Transplantation. 2024 Jan 1;108(1):137-147. doi: 10.1097/TP.0000000000004647. Epub 2023 Dec 13. Transplantation. 2024. PMID: 37271872 Free PMC article. Review.
-
Functional Changes of T-Cell Subsets with Age and CMV Infection.Int J Mol Sci. 2021 Sep 15;22(18):9973. doi: 10.3390/ijms22189973. Int J Mol Sci. 2021. PMID: 34576140 Free PMC article.
-
The Impact of T-cell Aging on Alloimmunity and Inflammaging.Transplantation. 2024 Mar 1;108(3):634-642. doi: 10.1097/TP.0000000000004715. Epub 2023 Jun 30. Transplantation. 2024. PMID: 37389638 Free PMC article.
-
Editorial: Immunosenescence in organ transplantation.Front Transplant. 2024 Jun 14;3:1422358. doi: 10.3389/frtra.2024.1422358. eCollection 2024. Front Transplant. 2024. PMID: 38993765 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

