The lung microbiome in lung transplantation
- PMID: 34120840
- PMCID: PMC8335643
- DOI: 10.1016/j.healun.2021.04.014
The lung microbiome in lung transplantation
Abstract
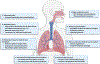
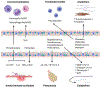
Culture-independent study of the lower respiratory tract after lung transplantation has enabled an understanding of the microbiome - that is, the collection of bacteria, fungi, and viruses, and their respective gene complement - in this niche. The lung has unique features as a microbial environment, with balanced entry from the upper respiratory tract, clearance, and local replication. There are many pressures impacting the microbiome after transplantation, including donor allograft factors, recipient host factors such as underlying disease and ongoing exposure to the microbe-rich upper respiratory tract, and transplantation-related immunosuppression, antimicrobials, and postsurgical changes. To date, we understand that the lung microbiome after transplant is dysbiotic; that is, it has higher biomass and altered composition compared to a healthy lung. Emerging data suggest that specific microbiome features may be linked to host responses, both immune and non-immune, and clinical outcomes such as chronic lung allograft dysfunction (CLAD), but many questions remain. The goal of this review is to put into context our burgeoning understanding of the lung microbiome in the postlung transplant patient, the interactions between microbiome and host, the role the microbiome may play in post-transplant complications, and critical outstanding research questions.
Keywords: chronic lung allograft dysfunction; hostmicrobe interactions; lung microbiome; lung transplantation.
Copyright © 2021 International Society for Heart and Lung Transplantation. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Upper Respiratory Dysbiosis with a Facultative-dominated Ecotype in Advanced Lung Disease and Dynamic Change after Lung Transplant.Ann Am Thorac Soc. 2019 Nov;16(11):1383-1391. doi: 10.1513/AnnalsATS.201904-299OC. Ann Am Thorac Soc. 2019. PMID: 31415219 Free PMC article.
-
The Human Respiratory Microbiome: Implications and Impact.Semin Respir Crit Care Med. 2018 Apr;39(2):199-212. doi: 10.1055/s-0037-1617441. Epub 2018 Mar 26. Semin Respir Crit Care Med. 2018. PMID: 29579771 Review.
-
The gut microbiome in end-stage lung disease and lung transplantation.mSystems. 2024 Jun 18;9(6):e0131223. doi: 10.1128/msystems.01312-23. Epub 2024 May 7. mSystems. 2024. PMID: 38712927 Free PMC article.
-
Dysbiosis of lower respiratory tract microbiome are associated with inflammation and microbial function variety.Respir Res. 2019 Dec 3;20(1):272. doi: 10.1186/s12931-019-1246-0. Respir Res. 2019. PMID: 31796027 Free PMC article.
-
The pulmonary microbiome: recent advances in understanding its role in chronic lung allograft dysfunction.Curr Opin Organ Transplant. 2022 Jun 1;27(3):217-221. doi: 10.1097/MOT.0000000000000956. Curr Opin Organ Transplant. 2022. PMID: 35649112 Review.
Cited by
-
Inquilinus limosus Bacteremia in Lung Transplant Recipient after SARS-CoV-2 Infection.Emerg Infect Dis. 2023 Mar;29(3):642-644. doi: 10.3201/eid2903.221564. Emerg Infect Dis. 2023. PMID: 36823767 Free PMC article.
-
The Lung Microbiome: A New Frontier for Lung and Brain Disease.Int J Mol Sci. 2023 Jan 21;24(3):2170. doi: 10.3390/ijms24032170. Int J Mol Sci. 2023. PMID: 36768494 Free PMC article. Review.
-
Hematopoietic Stem Cells Transplant (HSCT)-Related Chronic Pulmonary Diseases: An Overview.Children (Basel). 2023 Sep 11;10(9):1535. doi: 10.3390/children10091535. Children (Basel). 2023. PMID: 37761496 Free PMC article.
-
The Impact of Human Microbiotas in Hematopoietic Stem Cell and Organ Transplantation.Front Immunol. 2022 Jul 7;13:932228. doi: 10.3389/fimmu.2022.932228. eCollection 2022. Front Immunol. 2022. PMID: 35874759 Free PMC article. Review.
-
Alterations of lung microbiota in lung transplant recipients with pneumocystis jirovecii pneumonia.Respir Res. 2024 Mar 14;25(1):125. doi: 10.1186/s12931-024-02755-9. Respir Res. 2024. PMID: 38486264 Free PMC article.
References
-
- Chambers DC, Cherikh WS, Harhay MO, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart–lung transplantation Report—2019; Focus theme: Donor and recipient size match. J Hear Lung Transplant. 2019;38(10):1042–1055. doi:10.1016/j.healun.2019.08.001 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

