Phase 1 pharmacokinetics and safety study of extended duration dapivirine vaginal rings in the United States
- PMID: 34118115
- PMCID: PMC8196716
- DOI: 10.1002/jia2.25747
Phase 1 pharmacokinetics and safety study of extended duration dapivirine vaginal rings in the United States
Abstract
Introduction: Vaginal rings are a promising approach to provide a woman-centred, long-acting HIV prevention strategy. Prior trials of a 25 mg dapivirine (DPV) ring have shown a favourable safety profile and approximately 30% risk reduction of HIV-1 infection. Extended duration rings replaced every three months may encourage user adherence, improve health service efficiency and reduce cost overall. We evaluated safety, pharmacokinetics, adherence and acceptability of two three-month rings with different DPV dosages, compared with the monthly DPV ring.
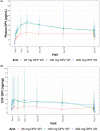
Methods: From December 2017 to October 2018, MTN-036/IPM-047 enrolled 49 HIV-negative participant in Birmingham, Alabama and San Francisco, California into a phase 1, randomized trial comparing two extended duration (three-month) rings (100 or 200 mg DPV) to a monthly 25 mg DPV ring, each used over 13 weeks, with follow-up completed in January 2019. Safety was assessed by recording adverse events (AEs). DPV concentrations were quantified in plasma, cervicovaginal fluid (CVF) and cervical tissue, at nominal timepoints. Geometric mean ratios (GMRs) relative to the comparator ring were estimated from a regression model.
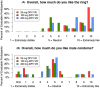
Results: There were no differences in the proportion of participants with grade ≥2 genitourinary AEs or grade ≥3 AEs in the extended duration versus monthly ring arms (p = 1.0). Plasma and CVF DPV concentrations were higher in the extended duration rings compared to the monthly ring. Plasma GMRs were 1.31 to 1.85 and 1.41 to 1.86 and CVF GMRs were 1.45 to 2.87 and 1.74 to 2.60 for the 100 and 200 mg ring respectively. Cervical tissue concentrations were consistently higher in the 200 mg ring (GMRs 2.36 to 3.97). The majority of participants (82%) were fully adherent (ring inserted at all times, with no product discontinuations/outages) with no differences between the monthly versus three-month rings. Most participants found the ring acceptable (median = 8 on 10-point Likert scale), with a greater proportion of participants reporting high acceptability (9 or 10) in the 25 mg arm (73%) compared with the 100 mg (25%) and 200 mg (44%) arms (p = 0.01 and p = 0.15 respectively).
Conclusions: The extended duration DPV rings were well-tolerated and achieved higher DPV concentrations compared with the monthly DPV ring. These findings support further evaluation of three-month DPV rings for HIV prevention.
Trial registration: ClinicalTrials.gov NCT03234400.
Keywords: dapivirine; microbicide; pharmacokinetics; pre-exposure prophylaxis; safety; vaginal ring.
© 2021 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of the International AIDS Society.
Figures
Similar articles
-
Phase 1 randomized trials to assess safety, pharmacokinetics, and vaginal bleeding associated with use of extended duration dapivirine and levonorgestrel vaginal rings.PLoS One. 2024 Jun 5;19(6):e0304552. doi: 10.1371/journal.pone.0304552. eCollection 2024. PLoS One. 2024. PMID: 38838028 Free PMC article. Clinical Trial.
-
Phase 2a Safety, Pharmacokinetics, and Acceptability of Dapivirine Vaginal Rings in US Postmenopausal Women.Clin Infect Dis. 2019 Mar 19;68(7):1144-1151. doi: 10.1093/cid/ciy654. Clin Infect Dis. 2019. PMID: 30289485 Free PMC article. Clinical Trial.
-
Phase 1 Safety, Pharmacokinetics, and Pharmacodynamics of Dapivirine and Maraviroc Vaginal Rings: A Double-Blind Randomized Trial.J Acquir Immune Defic Syndr. 2015 Nov 1;70(3):242-9. doi: 10.1097/QAI.0000000000000702. J Acquir Immune Defic Syndr. 2015. PMID: 26034880 Free PMC article. Clinical Trial.
-
An interdisciplinary framework for measuring and supporting adherence in HIV prevention trials of ARV-based vaginal rings.J Int AIDS Soc. 2014 Sep 8;17(3 Suppl 2):19158. doi: 10.7448/IAS.17.3.19158. eCollection 2014. J Int AIDS Soc. 2014. PMID: 25224617 Free PMC article. Review.
-
Development of dapivirine vaginal ring for HIV prevention.Antiviral Res. 2013 Dec;100 Suppl:S3-8. doi: 10.1016/j.antiviral.2013.09.025. Epub 2013 Nov 1. Antiviral Res. 2013. PMID: 24188702 Review.
Cited by
-
Users' Preferred Characteristics of Vaginal Rings for HIV Prevention: A Qualitative Analysis of Two Phase I Trials.AIDS Res Hum Retroviruses. 2022 Apr;38(4):313-326. doi: 10.1089/AID.2021.0077. Epub 2022 Feb 11. AIDS Res Hum Retroviruses. 2022. PMID: 34969254 Free PMC article. Clinical Trial.
-
HIV prevention: better choice for better coverage.J Int AIDS Soc. 2022 Jan;25(1):e25872. doi: 10.1002/jia2.25872. J Int AIDS Soc. 2022. PMID: 35030296 Free PMC article.
-
First-in-human study to assess the pharmacokinetics, tolerability, and safety of single-dose oxybutynin hydrochloride administered via a microprocessor-controlled intravaginal ring.Drug Deliv. 2023 Dec;30(1):2180113. doi: 10.1080/10717544.2023.2180113. Drug Deliv. 2023. PMID: 36815245 Free PMC article.
-
Phase 1 randomized trials to assess safety, pharmacokinetics, and vaginal bleeding associated with use of extended duration dapivirine and levonorgestrel vaginal rings.PLoS One. 2024 Jun 5;19(6):e0304552. doi: 10.1371/journal.pone.0304552. eCollection 2024. PLoS One. 2024. PMID: 38838028 Free PMC article. Clinical Trial.
-
Vaginal ring acceptability: A systematic review and meta-analysis of vaginal ring experiences from around the world.Contraception. 2022 Feb;106:16-33. doi: 10.1016/j.contraception.2021.10.001. Epub 2021 Oct 10. Contraception. 2022. PMID: 34644609 Free PMC article.
References
-
- UNAIDS . Women and HIV. A spotlight on adolescent girls and young women [cited 2020 Jul 29]. Available from: https://www.unaids.org/sites/default/files/media_asset/2019_women‐and‐hi...
-
- UNAIDS . Global HIV & AIDS statistics – 2020 fact sheet [cited 2020 Jul 29] https://www.unaids.org/en/resources/fact‐sheet
-
- Centers for Disease Control and Prevention . HIV and women [cited 2020 Jul 29]. Available from: https://www.cdc.gov/hiv/group/gender/women/index.html
-
- Hodges‐Mameletzis I, Fonner VA, Dalal S, Mugo N, Msimanga‐Radebe B, Baggaley R. Pre‐exposure prophylaxis for HIV prevention in women: current status and future directions. Drugs. 2019;79(12):1263–76. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

