HIV-1 Gag Recruits Oligomeric Vpr via Two Binding Sites in p6, but Both Mature p6 and Vpr Are Rapidly Lost upon Target Cell Entry
- PMID: 34106747
- PMCID: PMC8354229
- DOI: 10.1128/JVI.00554-21
HIV-1 Gag Recruits Oligomeric Vpr via Two Binding Sites in p6, but Both Mature p6 and Vpr Are Rapidly Lost upon Target Cell Entry
Abstract
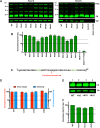
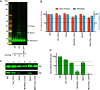
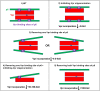
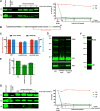
The p12 region of murine leukemia virus (MLV) Gag and the p6 region of HIV-1 Gag contain late domains required for virus budding. Additionally, the accessory protein Vpr is recruited into HIV particles via p6. Mature p12 is essential for early viral replication events, but the role of mature p6 in early replication is unknown. Using a proviral vector in which the gag and pol reading frames are uncoupled, we have performed the first alanine-scanning mutagenesis screens across p6 to probe its importance for early HIV-1 replication and to further understand its interaction with Vpr. The infectivity of our mutants suggests that, unlike p12, p6 is not important for early viral replication. Consistent with this, we observed that p6 is rapidly lost upon target cell entry in time course immunoblot experiments. By analyzing Vpr incorporation into p6 mutant virions, we identified that the 15-FRFG-18 and 41-LXXLF-45 motifs previously identified as putative Vpr-binding sites are important for Vpr recruitment but that the 34-ELY-36 motif also suggested to be a Vpr-binding site is dispensable. Additionally, disrupting Vpr oligomerization together with removing either binding motif in p6 reduced Vpr incorporation ∼25- to 50-fold more than inhibiting Vpr oligomerization alone and ∼10- to 25-fold more than deleting each p6 motif alone, implying that multivalency/avidity is important for the interaction. Interestingly, using immunoblotting and immunofluorescence, we observed that most Vpr is lost concomitantly with p6 during infection but that a small fraction remains associated with the viral capsid for several hours. This has implications for the function of Vpr in early replication. IMPORTANCE The p12 protein of MLV and the p6 protein of HIV-1 are both supplementary Gag cleavage products that carry proline-rich motifs that facilitate virus budding. Importantly, p12 has also been found to be essential for early viral replication events. However, while Vpr, the only accessory protein packaged into HIV-1 virions, is recruited via the p6 region of Gag, the function of both mature p6 and Vpr in early replication is unclear. Here, we have systematically mutated the p6 region of Gag and have studied the effects on HIV infectivity and Vpr packaging. We have also investigated what happens to p6 and Vpr during early infection. We show that, unlike p12, mature p6 is not required for early replication and that most of the mature p6 and the Vpr that it recruits are lost rapidly upon target cell entry. This has implications for the role of Vpr in target cells.
Keywords: Gag; Vpr; human immunodeficiency virus; p6; retroviruses.
Figures

Similar articles
-
The HIV-1 gag p6: a promising target for therapeutic intervention.Retrovirology. 2024 Jan 23;21(1):1. doi: 10.1186/s12977-024-00633-2. Retrovirology. 2024. PMID: 38263239 Free PMC article. Review.
-
The phosphorylation of HIV-1 Gag by atypical protein kinase C facilitates viral infectivity by promoting Vpr incorporation into virions.Retrovirology. 2014 Jan 22;11:9. doi: 10.1186/1742-4690-11-9. Retrovirology. 2014. PMID: 24447338 Free PMC article.
-
Structural studies of HIV-1 Gag p6ct and its interaction with Vpr determined by solution nuclear magnetic resonance.Biochemistry. 2009 Mar 24;48(11):2355-67. doi: 10.1021/bi801794v. Biochemistry. 2009. PMID: 19254034
-
Proline residues in human immunodeficiency virus type 1 p6(Gag) exert a cell type-dependent effect on viral replication and virion incorporation of Pol proteins.J Virol. 1999 Jun;73(6):4696-704. doi: 10.1128/JVI.73.6.4696-4704.1999. J Virol. 1999. PMID: 10233929 Free PMC article.
-
HIV-1 replication.Somat Cell Mol Genet. 2001 Nov;26(1-6):13-33. doi: 10.1023/a:1021070512287. Somat Cell Mol Genet. 2001. PMID: 12465460 Review.
Cited by
-
Advances in HIV-1 Assembly.Viruses. 2022 Feb 26;14(3):478. doi: 10.3390/v14030478. Viruses. 2022. PMID: 35336885 Free PMC article. Review.
-
HIV-1 Vpr drives a tissue residency-like phenotype during selective infection of resting memory T cells.Cell Rep. 2022 Apr 12;39(2):110650. doi: 10.1016/j.celrep.2022.110650. Cell Rep. 2022. PMID: 35417711 Free PMC article.
-
The HIV-1 gag p6: a promising target for therapeutic intervention.Retrovirology. 2024 Jan 23;21(1):1. doi: 10.1186/s12977-024-00633-2. Retrovirology. 2024. PMID: 38263239 Free PMC article. Review.
-
The HIV-1 Gag Protein Displays Extensive Functional and Structural Roles in Virus Replication and Infectivity.Int J Mol Sci. 2022 Jul 8;23(14):7569. doi: 10.3390/ijms23147569. Int J Mol Sci. 2022. PMID: 35886917 Free PMC article. Review.
-
Packaging and Uncoating of CRISPR/Cas Ribonucleoproteins for Efficient Gene Editing with Viral and Non-Viral Extracellular Nanoparticles.Viruses. 2023 Mar 6;15(3):690. doi: 10.3390/v15030690. Viruses. 2023. PMID: 36992399 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

