Munc18-1 Is Essential for Neuropeptide Secretion in Neurons
- PMID: 34103363
- PMCID: PMC8276746
- DOI: 10.1523/JNEUROSCI.3150-20.2021
Munc18-1 Is Essential for Neuropeptide Secretion in Neurons
Abstract
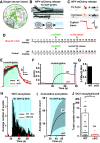
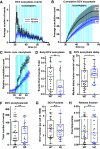
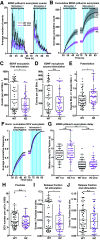
Neuropeptide secretion from dense-core vesicles (DCVs) controls many brain functions. Several components of the DCV exocytosis machinery have recently been identified, but the participation of a SEC1/MUNC18 (SM) protein has remained elusive. Here, we tested the ability of the three exocytic SM proteins expressed in the mammalian brain, MUNC18-1/2/3, to support neuropeptide secretion. We quantified DCV exocytosis at a single vesicle resolution on action potential (AP) train-stimulation in mouse CNS neurons (of unknown sex) using pHluorin-tagged and/or mCherry-tagged neuropeptide Y (NPY) or brain-derived neurotrophic factor (BDNF). Conditional inactivation of Munc18-1 abolished all DCV exocytosis. Expression of MUNC18-1, but not MUNC18-2 or MUNC18-3, supported DCV exocytosis in Munc18-1 null neurons. Heterozygous (HZ) inactivation of Munc18-1, as a model for reduced MUNC18-1 expression, impaired DCV exocytosis, especially during the initial phase of train-stimulation, when the release was maximal. These data show that neurons critically and selectively depend on MUNC18-1 for neuropeptide secretion. Impaired neuropeptide secretion may explain aspects of the behavioral and neurodevelopmental phenotypes that were observed in Munc18-1 HZ mice.SIGNIFICANCE STATEMENT Neuropeptide secretion from dense-core vesicles (DCVs) modulates synaptic transmission, sleep, appetite, cognition and mood. However, the mechanisms of DCV exocytosis are poorly characterized. Here, we identify MUNC18-1 as an essential component for neuropeptide secretion from DCVs. Paralogs MUNC18-2 or MUNC18-3 cannot compensate for MUNC18-1. MUNC18-1 is the first protein identified to be essential for both neuropeptide secretion and synaptic transmission. In heterozygous (HZ) Munc18-1 neurons, that have a 50% reduced MUNC18-1expression and model the human STXBP1 syndrome, DCV exocytosis is impaired, especially during the initial phase of train-stimulation, when the release is maximal. These data show that MUNC18-1 is essential for neuropeptide secretion and that impaired neuropeptide secretion on reduced MUNC18-1expression may contribute to the symptoms of STXBP1 syndrome.
Keywords: Munc18-1; dense-core vesicle; neuropeptide.
Copyright © 2021 the authors.
Figures



Similar articles
-
Rabphilin-3A negatively regulates neuropeptide release, through its SNAP25 interaction.Elife. 2024 Oct 16;13:RP95371. doi: 10.7554/eLife.95371. Elife. 2024. PMID: 39412498 Free PMC article.
-
Tomosyn affects dense core vesicle composition but not exocytosis in mammalian neurons.Elife. 2023 Sep 11;12:e85561. doi: 10.7554/eLife.85561. Elife. 2023. PMID: 37695731 Free PMC article.
-
Maximal Fusion Capacity and Efficient Replenishment of the Dense Core Vesicle Pool in Hippocampal Neurons.J Neurosci. 2023 Nov 8;43(45):7616-7625. doi: 10.1523/JNEUROSCI.2251-22.2023. Epub 2023 Oct 18. J Neurosci. 2023. PMID: 37852790 Free PMC article.
-
Mechanisms of exocytosis.Acta Physiol (Oxf). 2008 Feb;192(2):185-93. doi: 10.1111/j.1748-1716.2007.01803.x. Epub 2007 Nov 15. Acta Physiol (Oxf). 2008. PMID: 18005396 Review.
-
Role of Munc18-1 in synaptic vesicle and large dense-core vesicle secretion.Biochem Soc Trans. 2003 Aug;31(Pt 4):848-50. doi: 10.1042/bst0310848. Biochem Soc Trans. 2003. PMID: 12887319 Review.
Cited by
-
Activity-driven synaptic translocation of LGI1 controls excitatory neurotransmission.Cell Rep. 2024 May 28;43(5):114186. doi: 10.1016/j.celrep.2024.114186. Epub 2024 May 2. Cell Rep. 2024. PMID: 38700985 Free PMC article.
-
STXBP1-Related Disorders: Clinical Presentation, Molecular Function, Treatment, and Future Directions.Genes (Basel). 2023 Dec 5;14(12):2179. doi: 10.3390/genes14122179. Genes (Basel). 2023. PMID: 38137001 Free PMC article. Review.
-
The SNARE protein SNAP-25 is required for normal exocytosis at auditory hair cell ribbon synapses.iScience. 2022 Nov 22;25(12):105628. doi: 10.1016/j.isci.2022.105628. eCollection 2022 Dec 22. iScience. 2022. PMID: 36483015 Free PMC article.
-
Rabphilin-3A negatively regulates neuropeptide release, through its SNAP25 interaction.Elife. 2024 Oct 16;13:RP95371. doi: 10.7554/eLife.95371. Elife. 2024. PMID: 39412498 Free PMC article.
-
Distinct Alterations in Dendritic Spine Morphology in the Absence of β-Neurexins.Int J Mol Sci. 2024 Jan 20;25(2):1285. doi: 10.3390/ijms25021285. Int J Mol Sci. 2024. PMID: 38279285 Free PMC article.
References
-
- André T, Classen J, Brenner P, Betts MJ, Dörr B, Kreye S, Zuidinga B, Meijer M, Russell RB, Verhage M, Söllner TH (2020) The interaction of Munc18-1 helix 11 and 12 with the central region of the VAMP2 SNARE motif is essential for SNARE templating and synaptic transmission. eNeuro 7:ENEURO.0278-20.2020. 10.1523/ENEURO.0278-20.2020 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
